SimmonsŌĆōSmith reaction on:
[Wikipedia]
[Google]
[Amazon]
The SimmonsŌĆōSmith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an
 The SimmonsŌĆōSmith reaction is generally preferred over other methods of cyclopropanation, however it can be expensive due to the high cost of diiodomethane. Modifications involving cheaper alternatives have been developed, such as dibromomethane or
The SimmonsŌĆōSmith reaction is generally preferred over other methods of cyclopropanation, however it can be expensive due to the high cost of diiodomethane. Modifications involving cheaper alternatives have been developed, such as dibromomethane or
at ChemTube3D
:
 The
The
here
(java required). In another version of this reaction the ligand is based on salen and




SimmonsŌĆōSmith reaction at Organic Chemistry Portal
Name reactions {{DEFAULTSORT:Simmons-Smith reaction
alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbonŌĆōcarbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, ╬▒-olefins.
The Internationa ...
(or alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbonŌĆöcarbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
) to form a cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
Mechanism
Examples
Thus,cyclohexene
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an chemical intermediate, intermediate in the commercial synthesis of nylon.
Prod ...
, diiodomethane, and a zinc-copper couple (as iodomethylzinc iodide, ) yield norcarane (bicyclo .1.0eptane).
:diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
and zinc iodide. The reactivity of the system can also be increased by using the Furukawa modification, exchanging the zincŌĆæcopper couple for diethylzinc.
The SimmonsŌĆōSmith reaction is generally subject to steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
, and thus cyclopropanation usually takes place on the less hindered face. However, when a hydroxy substituent is present in the substrate in proximity to the double bond, the zinc coordinates with the hydroxy substituent, directing cyclopropanation ''cis'' to the hydroxyl group (which may not correspond to cyclopropanation of the sterically most accessible face of the double bond): An interactive 3D model of this reaction can be seeat ChemTube3D
:
Asymmetric SimmonsŌĆōSmith reaction
Although asymmetric cyclopropanation methods based on diazo compounds (the Metal-catalyzed cyclopropanations) exist since 1966, the asymmetric SimmonsŌĆōSmith reaction was introduced in 1992 with a reaction of cinnamyl alcohol with diethylzinc, diiodomethane and a chiral disulfonamide in dichloromethane: :hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group is a prerequisite serving as an anchor for zinc. An interactive 3D model of a similar reaction can be seehere
(java required). In another version of this reaction the ligand is based on salen and
Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
DIBAL is added:
:Scope and limitations
Achiral alkenes
The SimmonsŌĆōSmith reaction can be used to cyclopropanate simplealkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbonŌĆōcarbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, ╬▒-olefins.
The Internationa ...
without complications. Unfunctionalized achiral alkenes are best cyclopropanated with the Furukawa modification (see below), using and in 1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl ...
. Cyclopropanation of alkenes activated by electron donating groups proceed rapidly and easily. For example, enol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers incl ...
s like trimethylsilyloxy-substituted olefins are often used because of the high yields obtained.
Despite the electron-withdrawing nature of halides, many vinyl halides are also easily cyclopropanated, yielding fluoro-, bromo-, and iodo-substituted cyclopropanes.
The cyclopropanation of ''N''-substituted alkenes is made complicated by ''N''-alkylation as a competing pathway. This can be circumvented by adding a protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
to nitrogen, however the addition of electron-withdrawing groups decreases the nucleophilicity of the alkene, lowering yield. The use of highly electrophilic reagents such as , in place of , has been shown to increase yield in these cases.
Polyenes
Without the presence of a directing group on the olefin, very little chemoselectivity is observed. However, an alkene which is significantly more nucleophilic than any others will be highly favored. For example, cyclopropanation occurs highly selectively atenol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers incl ...
s.
Functional group compatibility
An important aspect of the SimmonsŌĆōSmith reaction that contributes to its wide usage is its ability to be used in the presence of many functional groups. Among others, the haloalkylzinc-mediated reaction is compatible with alkynes,alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
, ethers, aldehydes, ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
, carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
and derivatives, carbonates, sulfones, sulfonates, silanes, and stannanes. However, some side reactions are commonly observed.
Most side reactions occur due to the Lewis-acidity of the byproduct, . In reactions that produce acid-sensitive products, excess can be added to scavenge the that is formed, forming the less acidic . The reaction can also be quenched with pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
, which will scavenge and excess reagents.
Methylation
Methylation, in the chemistry, chemical sciences, is the addition of a methyl group on a substrate (chemistry), substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replac ...
of heteroatoms is also observed in the SimmonsŌĆōSmith reaction due to the electrophilicity of the zinc carbenoids. For example, the use of excess reagent for long reaction times almost always leads to the methylation of alcohols. Furthermore, and react with allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s to generate sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
ylides, which can subsequently undergo a 2,3-sigmatropic rearrangement, and will not cyclopropanate an alkene in the same molecule unless excess SimmonsŌĆōSmith reagent is used.Modifications
The SimmonsŌĆōSmith reaction is rarely used in it original form and a number of modifications to both the zinc reagent and carbenoid precursor have been developed and are more commonly employed.Furukawa modification
The Furukawa modification involves the replacement of the zinc-copper couple with dialkyl zinc, the most active of which was found to be . The modification was proposed in 1968 as a way to turn cationically polymerizableolefins
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbonŌĆōcarbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as ╬▒-olefins.
The International Union of P ...
such as vinyl ethers into their respective cyclopropanes. It has also been found to be especially useful for the cyclopropanation of carbohydrates, being far more reproducible than other methods. Like the unmodified reaction, the Furukawa-modified reaction is stereospecific, and is often much faster than the unmodified reaction. However, the reagent is pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
, and as such must be handled with care.

Charette modification
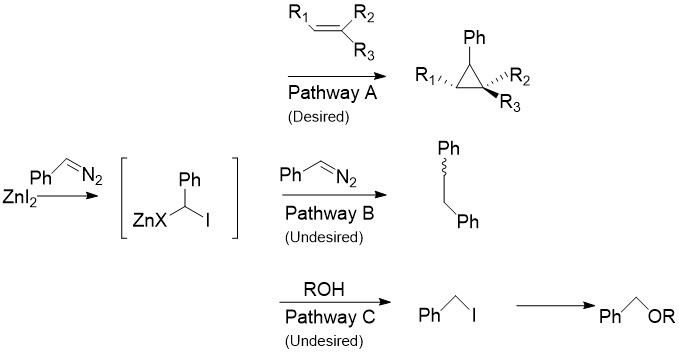
The Charette modification replaces the normally found in the SimmonsŌĆōSmith reaction with aryldiazo compounds, such as phenyldiazomethane, in Pathway A. Upon treatment with stoichiometric amounts of zinc halide, an organozinc compound similar to the carbenoid discussed above is produced. This can react with almost all alkenes and alkynes, including styrenes and alcohols. This is especially useful, as the unmodified Simmons-Smith is known to deprotonate alcohols. Unfortunately, as in Pathway B shown the intermediate can also react with the starting diazo compound, giving ''cis''- or ''trans''- 1,2-diphenylethene. Additionally, the intermediate can react with alcohols to produce iodophenylmethane, which can further undergo an SN2 reaction to produce , as in Pathway C.
Shi Modification
The highly electrophilic nature of the zinc carbenoid reduces the useful scope of the Simmons-Smith cyclopropanation to electron-rich alkenes and those bearing pendant coordinating groups, most commonly alcohols. In 1998, the Shi group identified a novel zinc carbenoid formed from diethylzinc,trifluoroacetic acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not ...
and diiodomethane of the form . This zinc carbenoid is far more nucleophilic and allows for reaction with unfunctionalized and electron-deficient alkenes, like vinyl boronates. A number of acidic modifiers have a similar effect, but trifluoroacetic acid is the most commonly used. The Shi modification of the cyclopropanation is also stereospecific. Further exploration of amino acids led to the development of an asymmetric variant of this cyclopropanation.

Non-zinc reagents
Although not commonly used, Simmons-Smith reagents that display similar reactive properties to those of zinc have been prepared from aluminum and samarium compounds in the presence of . With the use of these reagents, allylic alcohols and isolated olefins can be selectively cyclopropanated in the presence of each other. Iodo- or chloro- methylsamarium iodide in THF is an excellent reagent to selectively cyclopropanate the allylic alcohol, presumably directed bychelation
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
to the hydroxyl group. In contrast, use of dialkyl(iodomethyl)aluminum reagents in will selectively cyclopropanate the isolated olefin. The specificity of these reagents allow cyclopropanes to be placed in poly-unsaturated systems that zinc-based reagents will cyclopropanate fully and unselectively. For example, will cyclopropanate geraniol
Geraniol is a monoterpenoid and an alcohol. It is the primary component of citronella oil and is a primary component of rose oil and palmarosa oil. It is a colorless oil, although commercial samples can appear yellow. It has low solubility i ...
at the 6 position, while Sm/Hg, will cyclopropanate at the 2 position, as shown below. However, both reactions require near stoichiometric amounts of the starting metal compound, and Sm/Hg must be activated with the highly toxic .
Uses in synthesis
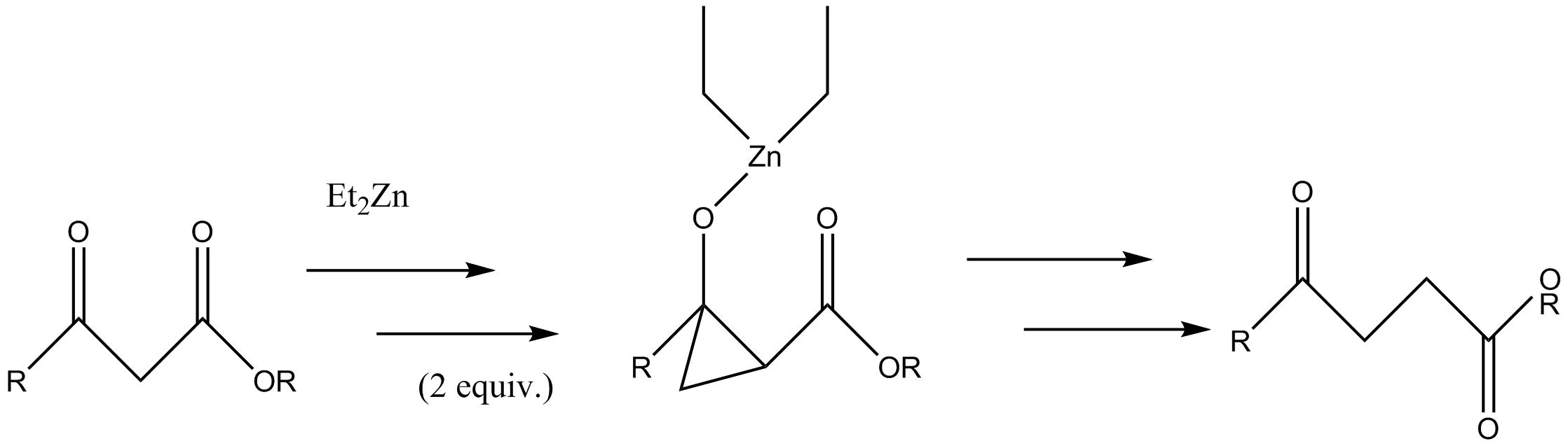
Most modern applications of the SimmonsŌĆōSmith reaction use the Furukawa modification. Especially relevant and reliable applications are listed below.Insertion to form ╬│-keto esters
A Furukawa-modified Simmons-Smith generatedcyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
intermediate is formed in the synthesis of ╬│-keto esters from ╬▓-keto esters. The Simmons-Smith reagent binds first to the carbonyl group
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes ...
and subsequently to the ╬▒-carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalentŌĆömeaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
of the pseudo-enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
that the first reaction forms. This second reagent forms the cyclopropyl intermediate which rapidly fragments into the product.

Formation of amido-spiro .2pentanes from allenamides
A Furukawa-modified SimmonsŌĆōSmith reaction cyclopropanates bothdouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s in an allenamide to form amido-spiro .2cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
s, featuring two cyclopropyl rings which share one carbon. The product of monocyclopropanation is also formed.
Natural product synthesis
Cyclopropanation reactions innatural product
A natural product is a natural compound or substance produced by a living organismŌĆöthat is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
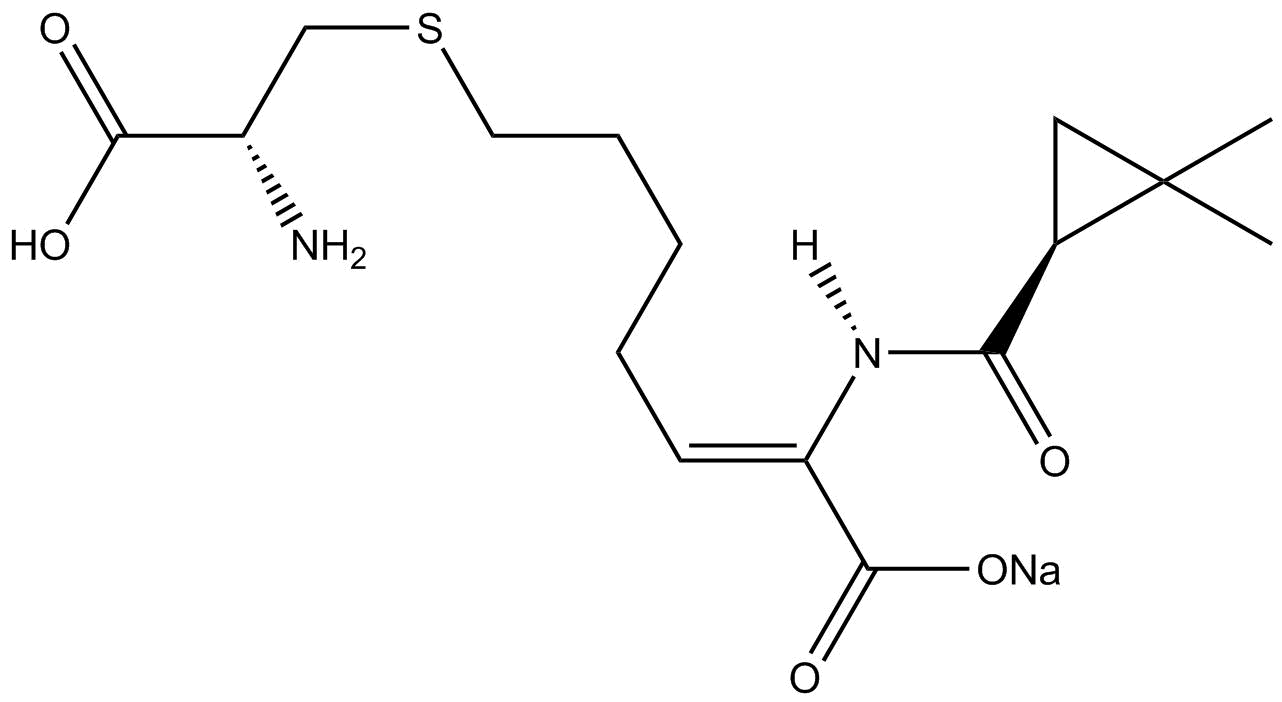
have been reviewed. The ╬▓-lactamase inhibitor Cilastatin provides an instructive example of Simmons-Smith reactivity in natural products synthesis. An allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
on the starting material is Simmons-Smith cyclopropanated, and the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
is subsequently deprotected via ozonolysis to form the precursor.


Pharmaceutical Synthesis
The SimmonsŌĆōSmith reaction is used in the syntheses of GSK1360707F, ropanicant and Onglyza (Saxagliptan).References
External links
SimmonsŌĆōSmith reaction at Organic Chemistry Portal
Name reactions {{DEFAULTSORT:Simmons-Smith reaction