Sharpless epoxidation on:
[Wikipedia]
[Google]
[Amazon]
The Sharpless epoxidation reaction is an  2,3-Epoxyalcohols can be converted into
2,3-Epoxyalcohols can be converted into
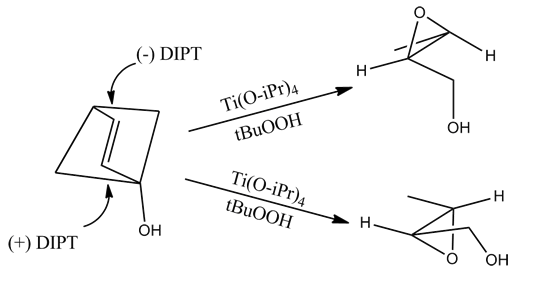 However, this method incorrectly predicts the product of allylic 1,2-diols.
However, this method incorrectly predicts the product of allylic 1,2-diols.


 As one of the few highly enantioselective reactions during its time, many manipulations of the 2,3-epoxyalcohols have been developed.
The Sharpless epoxidation has been used for the total synthesis of various
As one of the few highly enantioselective reactions during its time, many manipulations of the 2,3-epoxyalcohols have been developed.
The Sharpless epoxidation has been used for the total synthesis of various
Sharpless Asymmetric Epoxidation Reaction
{{DEFAULTSORT:Sharpless Epoxidation Epoxidation reactions Organic redox reactions Name reactions Epoxides Catalysis
enantioselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
is ''tert''-butyl hydroperoxide. The method relies on a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
formed from titanium tetra(isopropoxide) and diethyl tartrate.
diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting gro ...
s, aminoalcohols, and ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s. The reactants for the Sharpless epoxidation are commercially available and relatively inexpensive.
K. Barry Sharpless published a paper on the reaction in 1980 and was awarded the 2001 Nobel Prize in Chemistry for this and related work on asymmetric oxidations. The prize was shared with William S. Knowles and Ryōji Noyori.
Catalyst
5–10 mol% of the catalyst is typical. The presence of 3Åmolecular sieve
A molecular sieve is a material with pores of uniform size comparable to that of individual molecules, linking the interior of the solid to its exterior. These materials embody the molecular sieve effect, in which molecules larger than the pore ...
s (3Å MS) is necessary. The structure of the catalyst is uncertain although it is thought to be a dimer of [].
Selectivity
The epoxidation of allylic alcohols is a well-utilized conversion in fine chemical synthesis. The chirality of the product of a Sharpless epoxidation is sometimes predicted with the followingmnemonic
A mnemonic device ( ), memory trick or memory device is any learning technique that aids information retention or retrieval in the human memory, often by associating the information with something that is easier to remember.
It makes use of e ...
. A rectangle is drawn around the double bond in the same plane as the carbons of the double bond (the ''xy-plane''), with the allylic alcohol in the bottom right corner and the other substituents in their appropriate corners. In this orientation, the (−) diester tartrate preferentially interacts with the top half of the molecule, and the (+) diester tartrate preferentially interacts with the bottom half of the molecule. This model seems to be valid despite substitution on the olefin. Selectivity decreases with larger R1, but increases with larger R2 and R3 (see introduction).
 However, this method incorrectly predicts the product of allylic 1,2-diols.
However, this method incorrectly predicts the product of allylic 1,2-diols.

Kinetic resolution
The Sharpless epoxidation can also givekinetic resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, re ...
of a racemic mixture of secondary 2,3-epoxyalcohols. While the yield of a kinetic resolution process cannot be higher than 50%, the enantiomeric excess approaches 100% in some reactions.

Synthetic utility
The Sharpless epoxidation is viable with a large range of primary and secondary alkenic alcohols. Furthermore, with the exception noted above, a given dialkyl tartrate will preferentially add to the same face independent of the substitution on thealkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
.To demonstrate the synthetic utility of the Sharpless epoxidation, the Sharpless group created synthetic intermediates of various natural products: methymycin, erythromycin
Erythromycin is an antibiotic used for the treatment of a number of bacterial infections. This includes respiratory tract infections, skin infections, chlamydia infections, pelvic inflammatory disease, and syphilis. It may also be used ...
, leukotriene
Leukotrienes are a family of eicosanoid inflammation, inflammatory mediators produced in leukocytes by the redox, oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxyg ...
C-1, and (+)- disparlure.
 As one of the few highly enantioselective reactions during its time, many manipulations of the 2,3-epoxyalcohols have been developed.
The Sharpless epoxidation has been used for the total synthesis of various
As one of the few highly enantioselective reactions during its time, many manipulations of the 2,3-epoxyalcohols have been developed.
The Sharpless epoxidation has been used for the total synthesis of various saccharides
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ma ...
, terpenes
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
, leukotrienes
Leukotrienes are a family of eicosanoid inflammation, inflammatory mediators produced in leukocytes by the redox, oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxyg ...
, pheromones
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavi ...
, and antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
s.
The main drawback of this protocol is the necessity of the presence of an allylic alcohol. The Jacobsen epoxidation, an alternative method to enantioselectively oxidise alkenes, overcomes this issue and tolerates a wider array of functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s. For specifically glycidic epoxides, the Jørgensen-Córdova epoxidation avoids the need to reduce the carbonyl and then reoxidize, and has more efficient catalyst turnover.
References of historic interest
* *See also
* Asymmetric catalytic oxidation * Juliá–Colonna epoxidation — for enones * Jacobsen epoxidation — for unfunctionalized alkenesReferences
External links
Sharpless Asymmetric Epoxidation Reaction
{{DEFAULTSORT:Sharpless Epoxidation Epoxidation reactions Organic redox reactions Name reactions Epoxides Catalysis