Selinexor on:
[Wikipedia]
[Google]
[Amazon]
Selinexor sold under the brand name Xpovio among others, is a selective inhibitor of nuclear export used as an
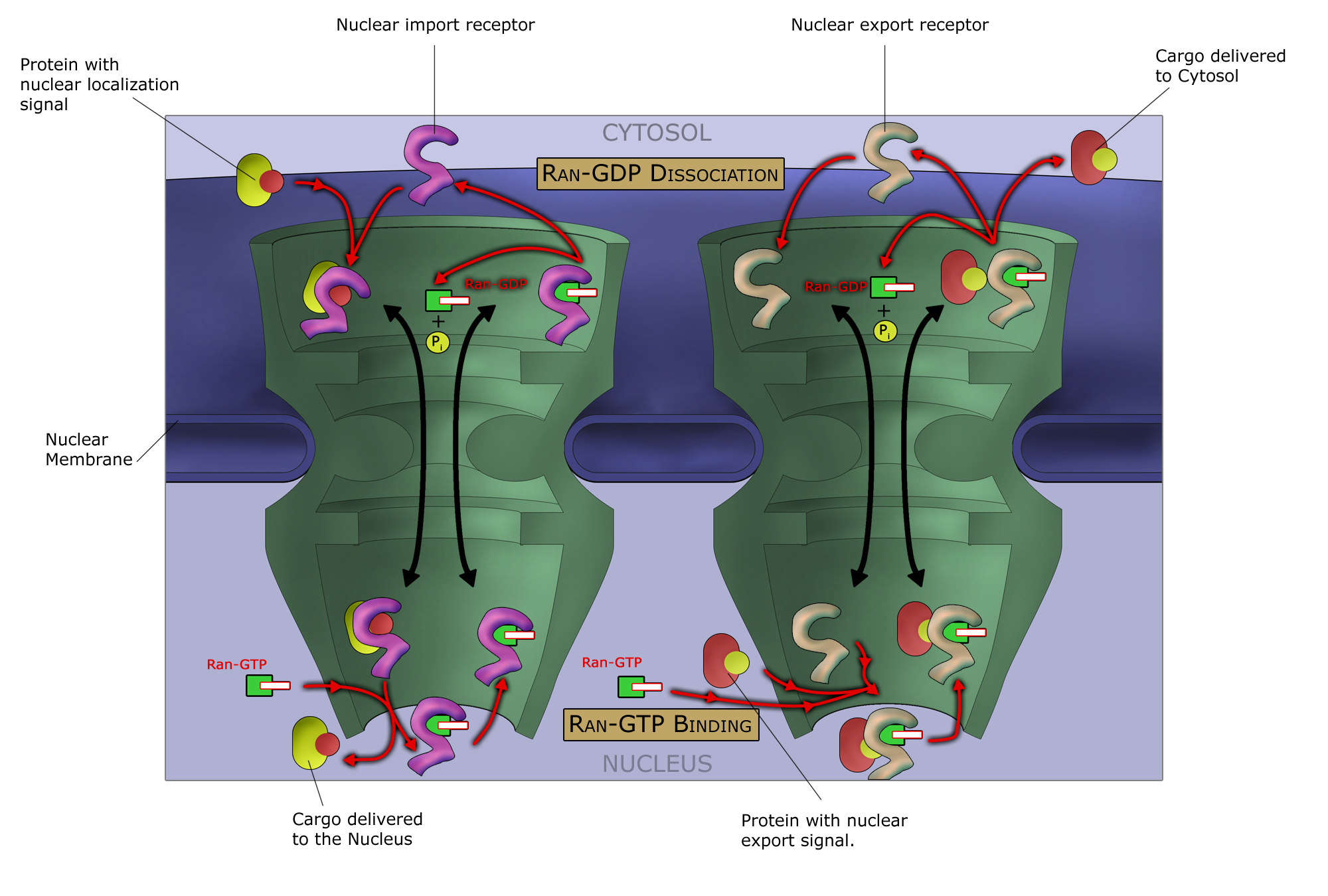 Like other selective inhibitors of nuclear export (SINEs), selinexor works by binding to exportin 1 (also known as XPO1 or CRM1). XPO1 is a
Like other selective inhibitors of nuclear export (SINEs), selinexor works by binding to exportin 1 (also known as XPO1 or CRM1). XPO1 is a
LVHN Among Early Participants to Test Cancer Drug as COVID-19 Treatment
, Lehigh Valley Hospital–Cedar Crest (LVHN) website, 7 May 2020 In this phase 2 randomized placebo-controlled single-blind trial named XPORT-CoV-1001 with a total of 190 participants with severe COVID-19, treatment with selinexor resulted in higher mortality (16% vs. 9%) and more serious adverse events (23% vs. 16%) than placebo.
anti-cancer medication
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs ( chemotherapeutic agents or alkylating agents) in a standard regimen. Chemotherapy may be given with a cu ...
. It works by blocking the action of exportin 1 and thus blocking the transport
Transport (in British English) or transportation (in American English) is the intentional Motion, movement of humans, animals, and cargo, goods from one location to another. Mode of transport, Modes of transport include aviation, air, land tr ...
of several proteins involved in cancer-cell growth from the cell nucleus
The cell nucleus (; : nuclei) is a membrane-bound organelle found in eukaryote, eukaryotic cell (biology), cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have #Anucleated_cells, ...
to the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
, which ultimately arrests the cell cycle
The cell cycle, or cell-division cycle, is the sequential series of events that take place in a cell (biology), cell that causes it to divide into two daughter cells. These events include the growth of the cell, duplication of its DNA (DNA re ...
and leads to apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
. It is the first drug with this mechanism of action
In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical Drug interaction, interaction through which a Medication, drug substance produces its pharmacological effect. A mechanism of action usually includes mention o ...
.
The most common side effects include nausea (feeling sick), vomiting, decreased appetite, weight loss, diarrhea, tiredness, thrombocytopenia (low blood-platelet counts), anaemia (low red-blood cell counts), low levels of white blood cells and hyponatraemia (low blood sodium levels).
Selinexor was granted accelerated approval by the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA) in July 2019, for use in combination with the corticosteroid dexamethasone
Dexamethasone is a fluorinated glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive pulmonary disease (COPD), croup, brain swelling, eye pain following eye su ...
for the treatment of adults with relapsed refractory multiple myeloma (RRMM) who have received at least four prior therapies and whose disease is resistant to several other forms of treatment, including at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody. In December 2020, selinexor was approved by the FDA in combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy. In clinical trials, it was associated with a high incidence of severe side effects, including low platelet counts and low blood sodium levels.
The U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA) considers it to be a first-in-class medication
A first-in-class medication is a prototype drug that uses a "new and unique mechanism of action" to treat a particular medical condition. While the Food and Drug Administration's Center for Drug Evaluation and Research tracks first-in-class medic ...
. Selinexor was approved for medical use in the European Union in March 2021.
Medical uses
Selinexor is approved in combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy. Selinexor is also approved for use in combination with the steroiddexamethasone
Dexamethasone is a fluorinated glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive pulmonary disease (COPD), croup, brain swelling, eye pain following eye su ...
in people with relapsed or refractory multiple myeloma
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibody, antibodies. Often, no symptoms are noticed initially. As it progresses, bone ...
who have received at least four prior therapies and whose disease is refractory to at least two proteosome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody (so-called "quad-refractory" or "penta-refractory" myeloma), for whom no other treatment options are available. It is the first drug to be approved for this indication.
In June 2020, the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA) approved an additional indication for selinexor to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy.
In the European Union, selinexor is indicated in combination with dexamethasone for the treatment of multiple myeloma in adults who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.
Adverse effects
In the clinical study (the BOSTON study) used to support FDA approval in patients with multiple myeloma after at least one prior therapy (once-weekly selinexor in combination with once-weekly bortezomib and dexamethasone),the most common adverse reactions were cytopenias, along with gastrointestinal and constitutional symptoms and were consistent with those previously reported from other selinexor studies. Most adverse reactions were manageable with dose modifications and/or standard supportive care. The most common non-hematologic adverse reactions were fatigue (59%), nausea (50%), decreased appetite (35%), and diarrhea (32%) and were mostly Grade 1 and 2 events. The most common Grade 3 and 4 adverse reactions were thrombocytopenia (43%), lymphopenia (38%), fatigue (28%) and anemia (17%). The most common adverse reactions (incidence ≥20%) in people with diffuse large B-cell lymphoma (DLBCL), excluding laboratory abnormalities, were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia. Grade 3-4 laboratory abnormalities in ≥15% were thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia. Serious adverse reactions occurred in 46% of people, most often from infection. Thrombocytopenia was the leading cause of dose modifications. Gastrointestinal toxicity developed in 80% of people and any grade hyponatremia developed in 61%. Central neurological adverse reactions occurred in 25% of people, including dizziness and mental status changes. The prescribing information provides warnings and precautions for thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, and embryo-fetal toxicity.Mechanism of action
 Like other selective inhibitors of nuclear export (SINEs), selinexor works by binding to exportin 1 (also known as XPO1 or CRM1). XPO1 is a
Like other selective inhibitors of nuclear export (SINEs), selinexor works by binding to exportin 1 (also known as XPO1 or CRM1). XPO1 is a karyopherin
Karyopherins are protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme c ...
which performs nuclear transport Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecule ...
of several proteins, including tumor suppressor
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell (biology), cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results ...
s, oncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.
s, and proteins involved in governing cell growth, from the cell nucleus
The cell nucleus (; : nuclei) is a membrane-bound organelle found in eukaryote, eukaryotic cell (biology), cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have #Anucleated_cells, ...
to the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
; it is often overexpressed and its function misregulated in several types of cancer. By inhibiting the XPO1 protein, SINEs lead to a buildup of tumor suppressors in the nucleus of malignant cells and reduce levels of oncogene products which drive cell proliferation. This ultimately leads to cell cycle arrest and death of cancer cells by apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
. ''In vitro'', this effect appeared to spare normal (non-malignant) cells.
Inhibiting XPO1 affects many different cells in the body which may explain the incidence of adverse reactions to selinexor. Thrombocytopenia, for example, is a mechanistic and dose-dependent effect, occurring because selinexor causes a buildup of the transcription factor STAT3
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor which in humans is encoded by the ''STAT3'' gene. It is a member of the STAT protein family.
Function
STAT3 is a member of the STAT protein family. In respon ...
in the nucleus of hematopoietic stem cell
Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the ...
s, preventing their differentiation into mature megakaryocyte
A megakaryocyte () is a large bone marrow cell with a lobation, lobated nucleus that produces blood platelets (thrombocytes), which are necessary for normal blood coagulation, clotting. In humans, megakaryocytes usually account for 1 out of 10,00 ...
s (platelet-producing cells) and thus slowing production of new platelets.
Chemistry
Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to acysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
residue in the nuclear export signal groove of exportin 1. Although this bond is covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
, it is slowly reversible.
History
Selinexor was developed by Karyopharm Therapeutics, a pharmaceutical company focused on the development of drugs that targetnuclear transport Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecule ...
. It was approved in the United States in July 2019, on the basis of a single-arm Phase IIb clinical trial. The FDA decided to grant accelerated approval despite a previous recommendation from an FDA Advisory Committee Panel which had voted 8–5 to delay approving the drug until the results from an ongoing Phase III study were known.
Selinexor in combination with dexamethasone was granted accelerated approval and was granted orphan drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by th ...
designation. The FDA granted the approval of Xpovio to Karyopharm Therapeutics.
In June 2020, the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA) approved an additional indication for selinexor to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy.
Approval was based on SADAL (KCP-330-009; NCT02227251), a multicenter, single-arm, open-label trial in participants with DLBCL after two to five systemic regimens. Participants received selinexor 60 mg orally on days one and three of each week.
In December 2020, the FDA expanded selinexor's approved indication to include its combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy.
Society and culture
Legal status
On 28 January 2021, theCommittee for Medicinal Products for Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as the Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regar ...
(CHMP) of the European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products ...
(EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Nexpovio intended for the treatment of relapsed and refractory multiple myeloma. The applicant for this medicinal product is Karyopharm Europe GmbH. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. Selinexor was approved for medical use in the European Union in March 2021.
Research
Under the codename KPT-330, selinexor was tested in several preclinicalanimal model
An animal model (short for animal disease model) is a living, non-human, often genetic-engineered animal used during the research and investigation of human disease, for the purpose of better understanding the disease process without the risk of ha ...
s of cancer, including pancreatic cancer
Pancreatic cancer arises when cell (biology), cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a Neoplasm, mass. These cancerous cells have the malignant, ability to invade other parts of ...
, breast cancer
Breast cancer is a cancer that develops from breast tissue. Signs of breast cancer may include a Breast lump, lump in the breast, a change in breast shape, dimpling of the skin, Milk-rejection sign, milk rejection, fluid coming from the nipp ...
, non-small-cell lung cancer
Non-small-cell lung cancer (NSCLC), or non-small-cell lung carcinoma, is any type of epithelial lung cancer other than small-cell lung cancer (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitiv ...
, lymphoma
Lymphoma is a group of blood and lymph tumors that develop from lymphocytes (a type of white blood cell). The name typically refers to just the cancerous versions rather than all such tumours. Signs and symptoms may include enlarged lymph node ...
s, and acute and chronic leukemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
s. In humans, early clinical trials (phase I) have been conducted in non-Hodgkin lymphoma
Non-Hodgkin lymphoma (NHL), also known as non-Hodgkin's lymphoma, is a group of blood cancers that includes all types of lymphomas except Hodgkin lymphomas. Symptoms include enlarged lymph nodes, fever, night sweats, weight loss, and tiredn ...
, blast crisis, and a wide range of advanced or refractory solid tumors, including colon cancer
Colorectal cancer (CRC), also known as bowel cancer, colon cancer, or rectal cancer, is the development of cancer from the colon or rectum (parts of the large intestine). Signs and symptoms may include blood in the stool, a change in bowel ...
, head and neck cancer
Head and neck cancer is a general term encompassing multiple cancers that can develop in the head and neck region. These include cancers of the mouth, tongue, gums and lips (oral cancer), voice box ( laryngeal), throat ( nasopharyngeal, orophary ...
, melanoma
Melanoma is the most dangerous type of skin cancer; it develops from the melanin-producing cells known as melanocytes. It typically occurs in the skin, but may rarely occur in the mouth, intestines, or eye (uveal melanoma). In very rare case ...
, ovarian cancer
Ovarian cancer is a cancerous tumor of an ovary. It may originate from the ovary itself or more commonly from communicating nearby structures such as fallopian tubes or the inner lining of the abdomen. The ovary is made up of three different ...
, and prostate cancer
Prostate cancer is the neoplasm, uncontrolled growth of cells in the prostate, a gland in the male reproductive system below the bladder. Abnormal growth of the prostate tissue is usually detected through Screening (medicine), screening tests, ...
. Compassionate use in patients with acute myeloid leukemia
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with haematopoiesis, normal blood cell production. Sympt ...
has also been reported.
The pivotal clinical trial which served to support approval of selinexor for people with relapsed/refractory multiple myeloma was an open-label study of 122 patients known as the STORM trial. In all of the enrolled patients, patients had been treated with a median of seven prior treatment regimens including conventional chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy re ...
, targeted therapy
Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy (oncology), hormonal therapy and cytotoxic chemotherapy. As a form of molecular medici ...
with bortezomib, carfilzomib, lenalidomide, pomalidomide
Pomalidomide, sold under the brand names Pomalyst and Imnovid, is an anti-cancer medication used for the treatment of multiple myeloma and AIDS-related Kaposi sarcoma.
Pomalidomide was approved for medical use in the United States in February ...
, and a monoclonal antibody
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Monoclonal antibodie ...
( daratumumab or isatuximab); nearly all had also undergone hematopoietic stem cell transplantation
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood, in order to replicate inside a patient and produce ...
but had disease that continued to progress. The overall response rate was 26%, including two stringent complete responses; 39% of patients had a minimal response or better. The median duration of response was 4.4 months, median progression-free survival was 3.7 months, and median overall survival was 8.6 months.
As of 2019, phase I/II and III trials are ongoing, including the use of selinexor in other cancers and in combinations with other drugs used for multiple myeloma.
In November 2020, results from the multi-center, Phase III, randomized study (NCT03110562) which evaluated 402 participants with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy were published in The Lancet. The study was designed to compare the efficacy, safety and certain health-related quality of life parameters of once-weekly selinexor in combination with once-weekly Velcade® (bortezomib) plus low-dose dexamethasone (SVd) versus twice-weekly Velcade® plus low-dose dexamethasone (Vd). The primary endpoint of the study was progression-free survival (PFS) and key secondary endpoints included overall response rate (ORR), rate of peripheral neuropathy, and others. Additionally, the BOSTON study allowed for patients on the Vd control arm to crossover to the SVd arm following objective (quantitative) progression of disease verified by an Independent Review Committee (IRC). The BOSTON study was conducted at over 150 clinical sites internationally.
Although the study had one of the highest proportions of patients with high-risk cytogenetics (~50%) as compared with other Velcade-based studies in previously treated myeloma, the median PFS in the SVd arm was 13.93 months compared to 9.46 months in the Vd arm, representing a 4.47 month (47%) increase in median PFS (hazard ratio R0.70; p=0.0075). The SVd group also demonstrated a significantly greater ORR compared to the Vd group (76.4% vs. 62.3%, p=0.0012). Patients who had received only one prior line of therapy also demonstrated a higher ORR on the SVd arm as compared to the Vd arm (80.8% vs. 65.7%, p=0.0082). Importantly, SVd therapy compared to Vd therapy showed consistent PFS benefit and higher ORR across several important subgroups.
In 2020, selinexor underwent a clinical trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel v ...
for treatment of COVID-19
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the coronavirus SARS-CoV-2. In January 2020, the disease spread worldwide, resulting in the COVID-19 pandemic.
The symptoms of COVID‑19 can vary but often include fever ...
., Lehigh Valley Hospital–Cedar Crest (LVHN) website, 7 May 2020 In this phase 2 randomized placebo-controlled single-blind trial named XPORT-CoV-1001 with a total of 190 participants with severe COVID-19, treatment with selinexor resulted in higher mortality (16% vs. 9%) and more serious adverse events (23% vs. 16%) than placebo.
References
External links
* {{Portal bar , Medicine Antineoplastic drugs Hydrazides Orphan drugs Pyrazines Teratogens Triazoles Trifluoromethyl compounds