Reflection High-energy Electron Diffraction on:
[Wikipedia]
[Google]
[Amazon]
Reflection high-energy electron diffraction (RHEED) is a technique used to characterize the surface of

 Two types of diffraction contribute to RHEED patterns. Some incident electrons undergo a single,
Two types of diffraction contribute to RHEED patterns. Some incident electrons undergo a single,
 Users sometimes rotate the sample around an axis perpendicular to the sampling surface during RHEED experiments to create a RHEED pattern called the azimuthal plot. Rotating the sample changes the intensity of the diffracted beams due to their dependence on the azimuth angle. RHEED specialists characterize film morphologies by measuring the changes in beam intensity and comparing these changes to theoretical calculations, which can effectively model the dependence of the intensity of diffracted beams on the azimuth angle.
Users sometimes rotate the sample around an axis perpendicular to the sampling surface during RHEED experiments to create a RHEED pattern called the azimuthal plot. Rotating the sample changes the intensity of the diffracted beams due to their dependence on the azimuth angle. RHEED specialists characterize film morphologies by measuring the changes in beam intensity and comparing these changes to theoretical calculations, which can effectively model the dependence of the intensity of diffracted beams on the azimuth angle.
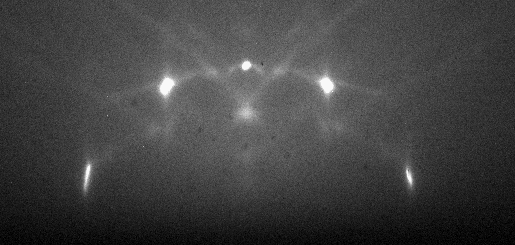
 Surface features and polycrystalline surfaces add complexity or change RHEED patterns from those from perfectly flat surfaces. Growing films, nucleating particles, crystal twinning, grains of varying size and adsorbed species add complicated diffraction conditions to those of a perfect surface. Superimposed patterns of the substrate and heterogeneous materials, complex interference patterns and degradation of the resolution are characteristic of complex surfaces or those partially covered with heterogeneous materials.
Surface features and polycrystalline surfaces add complexity or change RHEED patterns from those from perfectly flat surfaces. Growing films, nucleating particles, crystal twinning, grains of varying size and adsorbed species add complicated diffraction conditions to those of a perfect surface. Superimposed patterns of the substrate and heterogeneous materials, complex interference patterns and degradation of the resolution are characteristic of complex surfaces or those partially covered with heterogeneous materials.
 Each full period corresponds to formation of a single atomic layer thin film. The oscillation period is highly dependent on the material system, electron energy and incident angle, so researchers obtain empirical data to correlate the intensity oscillations and film coverage before using RHEED for monitoring film growth.
Video 1 depicts a metrology instrument recording the RHEED intensity oscillations and deposition rate for process control and analysis.
Each full period corresponds to formation of a single atomic layer thin film. The oscillation period is highly dependent on the material system, electron energy and incident angle, so researchers obtain empirical data to correlate the intensity oscillations and film coverage before using RHEED for monitoring film growth.
Video 1 depicts a metrology instrument recording the RHEED intensity oscillations and deposition rate for process control and analysis.
crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
materials. RHEED systems gather information only from the surface layer of the sample, which distinguishes RHEED from other materials characterization methods that also rely on diffraction of high-energy electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
. Transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a g ...
, another common electron diffraction
Electron diffraction refers to the bending of electron beams around atomic structures. This behaviour, typical for waves, is applicable to electrons due to the wave–particle duality stating that electrons behave as both particles and waves. Si ...
method samples the bulk of the sample due to the geometry of the system. Low-energy electron diffraction
Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low-energy electrons (30–200 eV) and observation of diffracted el ...
(LEED) is also surface sensitive, but LEED achieves surface sensitivity through the use of low energy electrons.
Introduction
A RHEED system requires an electron source (gun), photoluminescent detector screen and a sample with a clean surface, although modern RHEED systems have additional parts to optimize the technique. The electron gun generates a beam of electrons which strike the sample at a very small angle relative to the sample surface. Incident electrons diffract from atoms at the surface of the sample, and a small fraction of the diffracted electrons interfere constructively at specific angles and form regular patterns on the detector. The electrons interfere according to the position of atoms on the sample surface, so the diffraction pattern at the detector is a function of the sample surface. Figure 1 shows the most basic setup of a RHEED system.Surface diffraction
In the RHEED setup, only atoms at the sample surface contribute to the RHEED pattern. The glancing angle of incident electrons allows them to escape the bulk of the sample and to reach the detector. Atoms at the sample surface diffract (scatter) the incident electrons due to the wavelike properties of electrons. The diffracted electrons interfere constructively at specific angles according to the crystal structure and spacing of the atoms at the sample surface and the wavelength of the incident electrons. Some of the electron waves created by constructive interference collide with the detector, creating specific diffraction patterns according to the surface features of the sample. Users characterize the crystallography of the sample surface through analysis of the diffraction patterns. Figure 2 shows a RHEED pattern. Video 1 depicts a metrology instrument recording the RHEED intensity oscillations and deposition rate for process control and analysis. Two types of diffraction contribute to RHEED patterns. Some incident electrons undergo a single,
Two types of diffraction contribute to RHEED patterns. Some incident electrons undergo a single, elastic scattering
Elastic scattering is a form of particle scattering in scattering theory, nuclear physics and particle physics. In this process, the kinetic energy of a particle is conserved in the center-of-mass frame, but its direction of propagation is modi ...
event at the crystal surface, a process termed kinematic scattering. Dynamic scattering
The dynamical theory of diffraction describes the interaction of waves with a regular lattice. The wave fields traditionally described are X-rays, neutrons or electrons and the regular lattice are atomic crystal structures or nanometer-scale multi ...
occurs when electrons undergo multiple diffraction events in the crystal and lose some of their energy due to interactions with the sample. Users extract non-qualitative data from the kinematically diffracted electrons. These electrons account for the high intensity spots or rings common to RHEED patterns. RHEED users also analyze dynamically scattered electrons with complex techniques and models to gather quantitative information from RHEED patterns.
Kinematic scattering analysis
RHEED users constructEwald's sphere The Ewald sphere is a geometric construction used in electron, neutron, and X-ray crystallography which demonstrates the relationship between:
:* the wavevector of the incident and diffracted x-ray beams,
:* the diffraction angle for a given ref ...
s to find the crystallographic properties of the sample surface. Ewald's spheres show the allowed diffraction conditions for kinematically scattered electrons in a given RHEED setup. The diffraction pattern at the screen relates to the Ewald's sphere geometry, so RHEED users can directly calculate the reciprocal lattice of the sample with a RHEED pattern, the energy of the incident electrons and the distance from the detector to the sample. The user must relate the geometry and spacing of the spots of a perfect pattern to the Ewald's sphere in order to determine the reciprocal lattice of the sample surface.
The Ewald's sphere analysis is similar to that for bulk crystals, however the reciprocal lattice for the sample differs from that for a 3D material due to the surface sensitivity of the RHEED process. The reciprocal lattices of bulk crystals consist of a set of points in 3D space. However, only the first few layers of the material contribute to the diffraction in RHEED, so there are no diffraction conditions in the dimension perpendicular to the sample surface. Due to the lack of a third diffracting condition, the reciprocal lattice of a crystal surface is a series of infinite rods extending perpendicular to the sample's surface. These rods originate at the conventional 2D reciprocal lattice points of the sample's surface.
The Ewald's sphere is centered on the sample surface with a radius equal to the magnitude of the wavevector of the incident electrons,
where λ is the electrons' de Broglie wavelength
Matter waves are a central part of the theory of quantum mechanics, being an example of wave–particle duality. All matter exhibits wave-like behavior. For example, a beam of electrons can be diffracted just like a beam of light or a water wave ...
.
Diffraction conditions are satisfied where the rods of reciprocal lattice intersect the Ewald's sphere. Therefore, the magnitude of a vector from the origin of the Ewald's sphere to the intersection of any reciprocal lattice rods is equal in magnitude to that of the incident beam. This is expressed as
(2)
Here, khl is the wave vector of the elastically diffracted electrons of the order (hl) at any intersection of reciprocal lattice rods with Ewald's sphere
The projections of the two vectors onto the plane of the sample's surface differ by a reciprocal lattice vector Ghl,
(3)
Figure 3 shows the construction of the Ewald's sphere and provides examples of the G, khl and ki vectors.
Many of the reciprocal lattice rods meet the diffraction condition, however the RHEED system is designed such that only the low orders of diffraction are incident on the detector. The RHEED pattern at the detector is a projection only of the k vectors that are within the angular range that contains the detector. The size and position of the detector determine which of the diffracted electrons are within the angular range that reaches the detector, so the geometry of the RHEED pattern can be related back to the geometry of the reciprocal lattice of the sample surface through use of trigonometric relations and the distance from the sample to detector.
The k vectors are labeled such that the vector k00 that forms the smallest angle with the sample surface is called the 0th order beam. The 0th order beam is also known as the specular beam. Each successive intersection of a rod and the sphere further from the sample surface is labeled as a higher order reflection.
Because of the way the center of the Ewald's sphere is positioned, the specular beam forms the same angle with the substrate as the incident electron beam. The specular point has the greatest intensity on a RHEED pattern and is labeled as the (00) point by convention. The other points on the RHEED pattern are indexed according to the reflection order they project.
The radius of the Ewald's sphere is much larger than the spacing between reciprocal lattice rods because the incident beam has a very short wavelength due to its high-energy electrons. Rows of reciprocal lattice rods actually intersect the Ewald's sphere as an approximate plane because identical rows of parallel reciprocal lattice rods sit directly in front and behind the single row shown. Figure 3 shows a cross sectional view of a single row of reciprocal lattice rods filling of the diffraction conditions. The reciprocal lattice rods in Figure 3 show the end on view of these planes, which are perpendicular to the computer screen in the figure.
The intersections of these effective planes with the Ewald's sphere forms circles, called Laue circles. The RHEED pattern is a collection of points on the perimeters of concentric Laue circles around the center point. However, interference effects between the diffracted electrons still yield strong intensities at single points on each Laue circle. Figure 4 shows the intersection of one of these planes with the Ewald's Sphere.
The azimuthal angle affects the geometry and intensity of RHEED patterns. The azimuthal angle is the angle at which the incident electrons intersect the ordered crystal lattice on the surface of the sample. Most RHEED systems are equipped with a sample holder that can rotate the crystal around an axis perpendicular to the sample surface. RHEED users rotate the sample to optimize the intensity profiles of patterns. Users generally index at least 2 RHEED scans at different azimuth angles for reliable characterization of the crystal's surface structure. Figure 5 shows a schematic diagram of an electron beam incident on the sample at different azimuth angles.
Dynamic scattering analysis
The dynamically, or inelastically, scattered electrons provide several types of information about the sample as well. The brightness or intensity at a point on the detector depends on dynamic scattering, so all analysis involving the intensity must account for dynamic scattering. Some inelastically scattered electrons penetrate the bulk crystal and fulfill Bragg diffraction conditions. These inelastically scattered electrons can reach the detector to yield Kikuchi diffraction patterns, which are useful for calculating diffraction conditions. Kikuchi patterns are characterized by lines connecting the intense diffraction points on a RHEED pattern. Figure 6 shows a RHEED pattern with visibleKikuchi lines
Kikuchi lines are patterns of electrons formed by scattering. They pair up to form bands in electron diffraction from single crystal specimens, there to serve as "roads in orientation-space" for microscopists uncertain of what they are looking at ...
.

RHEED system requirements
Electron gun
Theelectron gun
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produces a narrow, collimated electron beam that has a precise kinetic energy. The largest use is in cathode-ray tubes (CRTs), used in nearly ...
is one of the most important piece of equipment in a RHEED system. The gun limits the resolution and testing limits of the system. Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
filaments are the primary electron source for the electron gun of most RHEED systems due to the low work function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
of tungsten. In the typical setup, the tungsten filament is the cathode and a positively biased anode draws electrons from the tip of the tungsten filament.
The magnitude of the anode bias determines the energy of the incident electrons. The optimal anode bias is dependent upon the type of information desired. At large incident angles, electrons with high energy can penetrate the surface of the sample and degrade the surface sensitivity of the instrument. However, the dimensions of the Laue zone
Max Theodor Felix von Laue (; 9 October 1879 – 24 April 1960) was a German physicist who received the Nobel Prize in Physics in 1914 for his discovery of the diffraction of X-rays by crystals.
In addition to his scientific endeavors with con ...
s are proportional to the inverse square of the electron energy meaning that more information is recorded at the detector at higher incident electron energies. For general surface characterization, the electron gun is operated the range of 10-30 keV.
In a typical RHEED setup, one magnetic and one electric field focus the incident beam of electrons. A negatively biased Wehnelt electrode positioned between the cathode filament and anode applies a small electric field, which focuses the electrons as they pass through the anode. An adjustable magnetic lens focuses the electrons onto the sample surface after they pass through the anode. A typical RHEED source has a focal length around 50 cm. The beam is focused to the smallest possible point at the detector rather than the sample surface so that the diffraction pattern has the best resolution.
Phosphor screens that exhibit photoluminescence are widely used as detectors. These detectors emit green light from areas where electrons hit their surface and are common to TEM as well. The detector screen is useful for aligning the pattern to an optimal position and intensity. CCD cameras capture the patterns to allow for digital analysis.
Sample surface
The sample surface must be extremely clean for effective RHEED experiments. Contaminants on the sample surface interfere with the electron beam and degrade the quality of the RHEED pattern. RHEED users employ two main techniques to create clean sample surfaces. Small samples can be cleaved in the vacuum chamber prior to RHEED analysis. The newly exposed, cleaved surface is analyzed. Large samples, or those that are not able to be cleaved prior to RHEED analysis can be coated with a passive oxide layer prior to analysis. Subsequent heat treatment under the vacuum of the RHEED chamber removes the oxide layer and exposes the clean sample surface.Vacuum requirements
Because gas molecules diffract electrons and affect the quality of the electron gun, RHEED experiments are performed under vacuum. The RHEED system must operate at a pressure low enough to prevent significant scattering of the electron beams by gas molecules in the chamber. At electron energies of 10keV, a chamber pressure of 10−5 mbar or lower is necessary to prevent significant scattering of electrons by the background gas. In practice, RHEED systems are operated under ultra high vacuums. The chamber pressure is minimized as much as possible in order to optimize the process. The vacuum conditions limit the types of materials and processes that can be monitored in situ with RHEED.RHEED patterns of real surfaces
Previous analysis focused only on diffraction from a perfectly flat surface of a crystal surface. However, non-flat surfaces add additional diffraction conditions to RHEED analysis. Streaked or elongated spots are common to RHEED patterns. As Fig 3 shows, the reciprocal lattice rods with the lowest orders intersect the Ewald sphere at very small angles, so the intersection between the rods and sphere is not a singular point if the sphere and rods have thickness. The incident electron beam diverges and electrons in the beam have a range of energies, so in practice, the Ewald sphere is not infinitely thin as it is theoretically modeled. The reciprocal lattice rods have a finite thickness as well, with their diameters dependent on the quality of the sample surface. Streaks appear in the place of perfect points when broadened rods intersect the Ewald sphere. Diffraction conditions are fulfilled over the entire intersection of the rods with the sphere, yielding elongated points or ‘streaks’ along the vertical axis of the RHEED pattern. In real cases, streaky RHEED patterns indicate a flat sample surface while the broadening of the streaks indicate small area of coherence on the surface. Surface features and polycrystalline surfaces add complexity or change RHEED patterns from those from perfectly flat surfaces. Growing films, nucleating particles, crystal twinning, grains of varying size and adsorbed species add complicated diffraction conditions to those of a perfect surface. Superimposed patterns of the substrate and heterogeneous materials, complex interference patterns and degradation of the resolution are characteristic of complex surfaces or those partially covered with heterogeneous materials.
Surface features and polycrystalline surfaces add complexity or change RHEED patterns from those from perfectly flat surfaces. Growing films, nucleating particles, crystal twinning, grains of varying size and adsorbed species add complicated diffraction conditions to those of a perfect surface. Superimposed patterns of the substrate and heterogeneous materials, complex interference patterns and degradation of the resolution are characteristic of complex surfaces or those partially covered with heterogeneous materials.
Specialized RHEED techniques
Film growth
RHEED is an extremely popular technique for monitoring the growth of thin films. In particular, RHEED is well suited for use withmolecular beam epitaxy
Molecular-beam epitaxy (MBE) is an epitaxy method for thin-film deposition of single crystals. MBE is widely used in the manufacture of semiconductor devices, including transistors, and it is considered one of the fundamental tools for the devel ...
(MBE), a process used to form high quality, ultrapure thin films under ultrahigh vacuum growth conditions. The intensities of individual spots on the RHEED pattern fluctuate in a periodic manner as a result of the relative surface coverage of the growing thin film. Figure 8 shows an example of the intensity fluctuating at a single RHEED point during MBE growth.
 Each full period corresponds to formation of a single atomic layer thin film. The oscillation period is highly dependent on the material system, electron energy and incident angle, so researchers obtain empirical data to correlate the intensity oscillations and film coverage before using RHEED for monitoring film growth.
Video 1 depicts a metrology instrument recording the RHEED intensity oscillations and deposition rate for process control and analysis.
Each full period corresponds to formation of a single atomic layer thin film. The oscillation period is highly dependent on the material system, electron energy and incident angle, so researchers obtain empirical data to correlate the intensity oscillations and film coverage before using RHEED for monitoring film growth.
Video 1 depicts a metrology instrument recording the RHEED intensity oscillations and deposition rate for process control and analysis.
RHEED-TRAXS
Reflection high energy electron diffraction - total reflection angle X-ray spectroscopy is a technique for monitoring the chemical composition of crystals. RHEED-TRAXS analyzes X-ray spectral lines emitted from a crystal as a result of electrons from a RHEED gun colliding with the surface. RHEED-TRAXS is preferential to X-ray microanalysis (XMA)(such as EDS and WDS) because the incidence angle of the electrons on the surface is very small, typically less than 5°. As a result, the electrons do not penetrate deeply into the crystal, meaning the X-ray emission is restricted to the top of the crystal, allowing for real-time, in-situ monitoring of surface stoichiometry. The experimental setup is fairly simple. Electrons are fired onto a sample causing X-ray emission. These X-rays are then detected using asilicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
-lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
Si-Li crystal placed behind beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form mi ...
windows, used to maintain vacuum.
MCP-RHEED
MCP-RHEED is a system in which anelectron beam
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to ele ...
is amplified by a micro-channel plate (MCP). This system consists of an electron gun
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produces a narrow, collimated electron beam that has a precise kinetic energy. The largest use is in cathode-ray tubes (CRTs), used in nearly ...
and an MCP equipped with a fluorescent
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
screen opposite to the electron gun. Because of the amplification, the intensity of the electron beam can be decreased by several orders of magnitude and the damage to the samples is diminished. This method is used to observe the growth of insulator crystals such as organic films and alkali halide
In chemistry, alkali metal halides, or alkali halides, are the family of inorganic compounds with the chemical formula MX, where M is an alkali metal and X is a halogen. These compounds are the often commercially significant sources of these metal ...
films, which are easily damaged by electron beams.
References
{{Reflist, 30emFurther reading
*Introduction to RHEED, A.S. Arrot, Ultrathin Magnetic Structures I, ''Springer-Verlag'', 1994, pp. 177–220 *A Review of the Geometrical Fundamentals of RHEED with Application to Silicon Surfaces, John E. Mahan, Kent M. Geib, G.Y. Robinson, and Robert G. Long, ''J.V.S.T.'', A 8, 1990, pp. 3692–3700 Crystallography Electron spectroscopy Diffraction Measuring instruments X-ray spectroscopy