Rotaxane Crystal Structure ChemComm Page493 2001 Commons on:
[Wikipedia]
[Google]
[Amazon]
 A rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a
A rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a
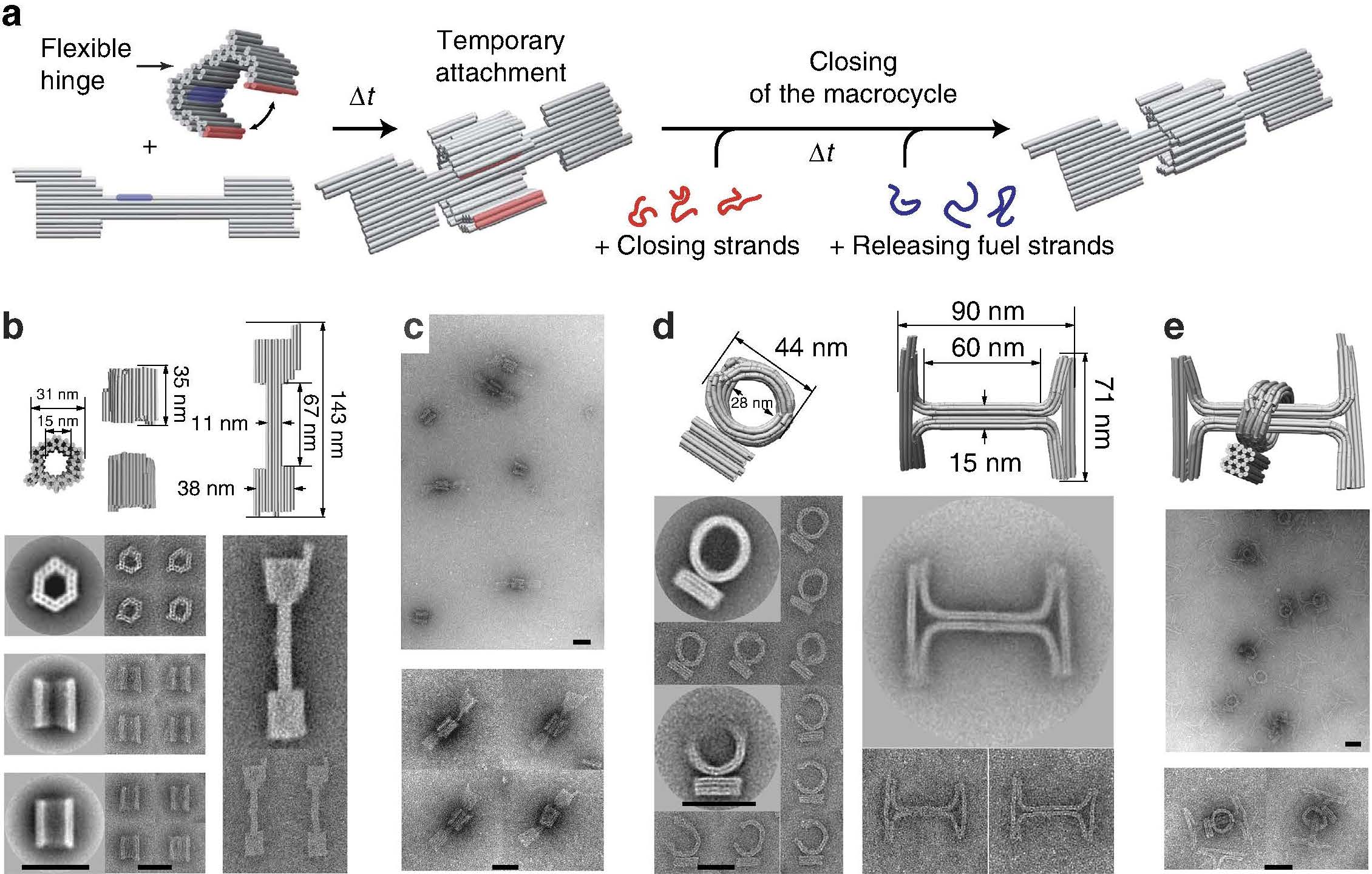
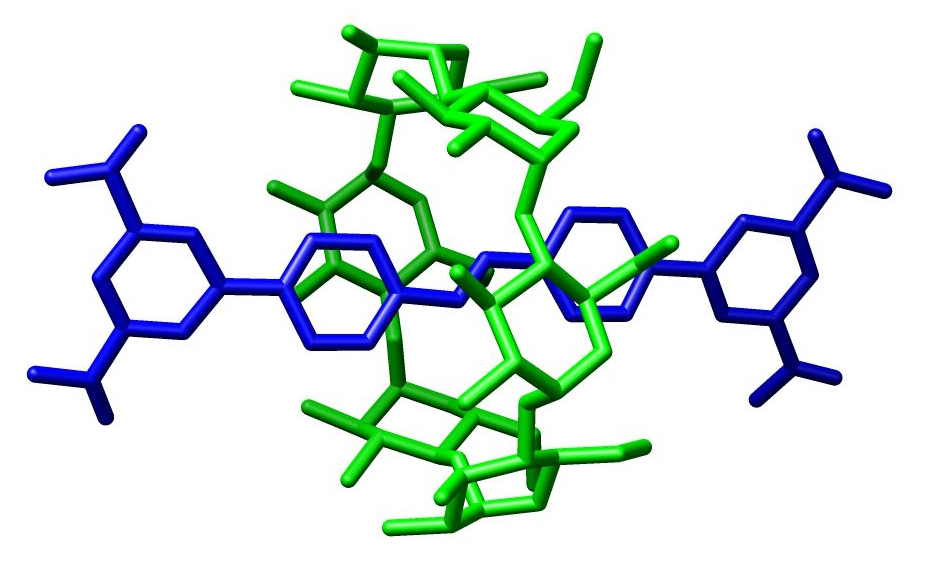
 A rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a
A rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
(see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called ''stoppers'') are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s.
Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenanes, has been focused on their efficient synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
or their utilization as artificial molecular machine
Molecular machines are a class of molecules typically described as an assembly of a discrete number of molecular components intended to produce mechanical movements in response to specific stimuli, mimicking macromolecular devices such as switch ...
s. However, examples of rotaxane substructure have been found in naturally occurring peptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Dalton (unit), Da or more are called proteins. Chains of fewer t ...
, including: cystine knot peptides, cyclotides or lasso-peptides such as microcin J25.
Synthesis
The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probability that if two halves of a dumbbell-shaped molecule were reacted in the presence of amacrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
that some small percentage would connect through the ring. To obtain a reasonable quantity of rotaxane, the macrocycle was attached to a solid-phase support and treated with both halves of the dumbbell 70 times and then severed from the support to give a 6% yield. However, the synthesis of rotaxanes has advanced significantly and efficient yields can be obtained by preorganizing the components utilizing hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
ing, metal coordination, hydrophobic forces, covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s, or coulombic interactions. The three most common strategies to synthesize rotaxane are "capping", "clipping", and "slipping", though others do exist. Recently, Leigh and co-workers described a new pathway to mechanically interlocked architectures involving a transition-metal center that can catalyse a reaction through the cavity of a macrocycle.

Capping
Synthesis via the capping method relies strongly upon a thermodynamically driven template effect; that is, the "thread" is held within the "macrocycle" by non-covalent interactions, for example rotaxinations with cyclodextrin macrocycles involve exploitation of the hydrophobic effect. This dynamic complex or pseudorotaxane is then converted to the rotaxane by reacting the ends of the threaded guest with large groups, preventing disassociation.Clipping
The clipping method is similar to the capping reaction except that in this case the dumbbell shaped molecule is complete and is bound to a partial macrocycle. The partial macrocycle then undergoes a ring closing reaction around the dumbbell-shaped molecule, forming the rotaxane.Slipping
The method of slipping is one which exploits the thermodynamic stability of the rotaxane. If the end groups of the dumbbell are an appropriate size it will be able to reversibly thread through the macrocycle at higher temperatures. By cooling the dynamic complex, it becomes kinetically trapped as a rotaxane at the lower temperature.Snapping
Snapping involves two separate parts of the thread, both containing a bulky group. one part of the thread is then threaded to the macrocycle, forming a semi rotaxane, and end is closed of by the other part of the thread forming the rotaxane."Active template" methodology
Leigh and co-workers recently began to explore a strategy in which template ions could also play an active role in promoting the crucial final covalent bond forming reaction that captures the interlocked structure (i.e., the metal has a dual function, acting as a template for entwining the precursors and catalyzing covalent bond formation between the reactants).Potential applications

Molecular machines
Rotaxane-based molecular machines have been of initial interest for their potential use inmolecular electronics
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. It provides a potential means to ...
as logic molecular switching elements and as molecular shuttles. These molecular machines
Molecular machines are a class of molecules typically described as an assembly of a discrete number of molecular components intended to produce mechanical movements in response to specific stimuli, mimicking macromolecule, macromolecular devices ...
are usually based on the movement of the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
on the dumbbell. The macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
can rotate around the axis of the dumbbell like a wheel and axle or it can slide along its axis from one site to another. Controlling the position of the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
allows the rotaxane to function as a molecular switch, with each possible location of the macrocycle corresponding to a different state. These rotaxane machines can be manipulated both by chemical and photochemical inputs. Rotaxane based systems have also been shown to function as molecular muscles. In 2009, there was a report of a "domino effect" from one extremity to the other in a Glycorotaxane Molecular Machine. In this case, the 4''C''1 or 1''C''4 chair-like conformation of the manno pyranoside stopper can be controlled, depending on the localization of the macrocycle. In 2012, unique pseudo-macrocycles consisting of double-lasso molecular machines (also called rotamacrocycles) were reported in Chem. Sci. These structures can be tightened or loosened depending on pH. A controllable jump rope movement was also observed in these new molecular machines.
Ultrastable dyes
Potential application as long-lasting dyes is based on the enhanced stability of the inner portion of the dumbbell-shaped molecule. Studies withcyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocycle, macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzyme, enzymatic conversion. They are used in ...
-protected rotaxane azo dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C l ...
s established this characteristic. More reactive squaraine dyes have also been shown to have enhanced stability by preventing nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
of the inner squaraine moiety. The enhanced stability of rotaxane dyes is attributed to the insulating effect of the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
, which is able to block interactions with other molecules.
Nanorecording
In a nanorecording application, a certain rotaxane is deposited as a Langmuir–Blodgett film onITO
Ito, Itō or Itoh may refer to:
Places
* Ito Island, an island of Milne Bay Province, Papua New Guinea
* Ito Airport, an airport in the Democratic Republic of the Congo
* Ito District, Wakayama, a district located in Wakayama Prefecture, Japa ...
-coated glass. When a positive voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
is applied with the tip of a scanning tunneling microscope
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
probe, the rotaxane rings in the tip area switch to a different part of the dumbbell and the resulting new conformation makes the molecules stick out 0.3 nanometer
330px, Different lengths as in respect to the Molecule">molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm), or nanometer (American spelling
Despite the va ...
from the surface. This height difference is sufficient for a memory dot. It is not yet known how to erase such a nanorecording film.
Nomenclature
Accepted nomenclature is to designate the number of components of the rotaxane in brackets as a prefix. Therefore, the rotaxane consisting of a single dumbbell-shaped axial molecule with a single macrocycle around its shaft is called a otaxane, and two cyanostar molecules around the central phosphate group of dialkylphosphate is a otaxane.See also
* Catenane * Mechanically interlocked molecular architecture * Molecular Borromean rings * Molecular knots * PolyrotaxaneReferences
{{reflist, 30em Supramolecular chemistry Molecular electronics Organic semiconductors Molecular topology Articles containing video clips