Raoult's law ( law) is a relation of
physical chemistry, with implications in
thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
. Proposed by French chemist
François-Marie Raoult in 1887,
it states that the
partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of each component of an
ideal mixture of ''liquids'' is equal to the
vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
of the pure component (liquid or solid) multiplied by its
mole fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the to ...
in the mixture. In consequence, the relative lowering of vapor pressure of a dilute solution of nonvolatile
solute is equal to the mole fraction of solute in the solution.
Mathematically, Raoult's law for a single component in an ideal solution is stated as
:
where
is the
partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of the component
in the gaseous mixture above the solution,
is the
equilibrium vapor pressure of the pure component
, and
is the mole fraction of the component
in the liquid or solid solution.
Where two volatile liquids A and B are mixed with each other to form a solution, the vapor phase consists of both components of the solution. Once the components in the solution have reached
equilibrium, the total vapor pressure of the solution can be determined by combining Raoult's law with
Dalton's law of partial pressures to give
:
In other words, the vapor pressure of the solution is the mole-weighted mean of the individual vapour pressures:
:
If a non-volatile solute B (it has zero vapor pressure, so does not
evaporate) is dissolved into a solvent A to form an ideal solution, the vapor pressure of the solution will be lower than that of the solvent. In an ideal solution of a nonvolatile solute, the decrease in vapor pressure is directly proportional to the mole fraction of solute:
:
:
If the solute associates or dissociates in the solution (such as an electrolyte/salt), the expression of the law includes the
van 't Hoff factor as a correction factor. That is, the mole fraction must be calculated using the actual number of particles in solution.
Principles
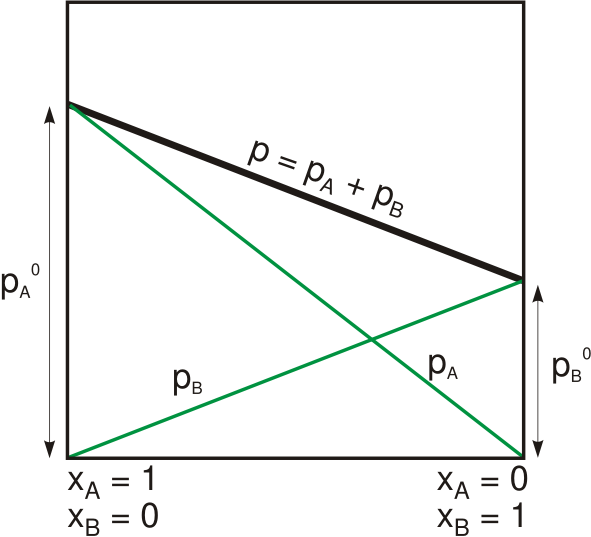
Raoult's law is a phenomenological relation that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules, and that their molar volumes are the same: the conditions of an ideal solution. This is analogous to the
ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
, which is a limiting law valid when the interactive forces between molecules approach zero, for example as the concentration approaches zero. Raoult's law is instead valid if the physical properties of the components are identical. The more similar the components are, the more their behavior approaches that described by Raoult's law. For example, if the two components differ only in
isotopic content, then Raoult's law is essentially exact.
Comparing measured vapor pressures to predicted values from Raoult's law provides information about the true relative strength of
intermolecular forces. If the vapor pressure is less than predicted (a negative deviation), fewer molecules of each component than expected have left the solution in the presence of the other component, indicating that the forces between unlike molecules are stronger. The converse is true for positive deviations.
For a solution of two liquids A and B, Raoult's law predicts that if no other gases are present, then the total vapor pressure
above the solution is equal to the weighted sum of the "pure" vapor pressures
and
of the two components. Thus the total pressure above the solution of A and B would be
:
Since the sum of the mole fractions is equal to one,
:
This is a linear function of the mole fraction
, as shown in the graph.
Thermodynamic considerations
Raoult's law was first observed empirically and led
François-Marie Raoult to postulate that the vapor pressure above an ideal mixture of liquids is equal to the sum of the vapor pressures of each component multiplied by its mole fraction.
Taking compliance with Raoult's Law as a defining characteristic of ideality in a solution, it is possible to
deduce that the
chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
of each component of the liquid is given by
:
where
is the chemical potential in the pure state and
is the mole fraction of component
in the ideal solution. From this equation, other thermodynamic properties of an ideal solution may be determined. If the assumption that the vapor follows the ideal gas law is added, Raoult's law may be derived as follows.
If the system is ideal, then, at
equilibrium, the chemical potential of each component
must be the same in the liquid and gas states. That is,
:
Substituting the formula for chemical potential gives
:
as the gas-phase mole fraction depends on its
fugacity,
, as a fraction of the pressure in the reference state,
.
The corresponding equation when the system consists purely of component
in equilibrium with its vapor is
:
Subtracting these equations and re-arranging leads to the result
:
For the ideal gas, pressure and fugacity are equal, so introducing simple
pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
s to this result yields Raoult's law:
:
Ideal mixing
An ideal solution would follow Raoult's law, but most solutions deviate from ideality. Interactions between gas molecules are typically quite small, especially if the vapor pressures are low. However, the interactions in a liquid are very strong. For a solution to be ideal, the interactions between unlike molecules must be of the same magnitude as those between like molecules. This approximation is only true when the different species are almost chemically identical. One can see that from considering the
Gibbs free energy change of mixing:
:
This is always negative, so mixing is spontaneous. However, the expression is, apart from a factor
, equal to the entropy of mixing. This leaves no room at all for an enthalpy effect and implies that
must be equal to zero, and this can only be true if the interactions between the molecules are indifferent.
It can be shown using the
Gibbs–Duhem equation that if Raoult's law holds over the entire concentration range
 Raoult's law is a phenomenological relation that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules, and that their molar volumes are the same: the conditions of an ideal solution. This is analogous to the
Raoult's law is a phenomenological relation that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules, and that their molar volumes are the same: the conditions of an ideal solution. This is analogous to the  Raoult's law is a phenomenological relation that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules, and that their molar volumes are the same: the conditions of an ideal solution. This is analogous to the
Raoult's law is a phenomenological relation that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules, and that their molar volumes are the same: the conditions of an ideal solution. This is analogous to the