Pyrrole Structure on:
[Wikipedia]
[Google]
[Amazon]
Pyrrole is a
 Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of cofactors and
Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of cofactors and

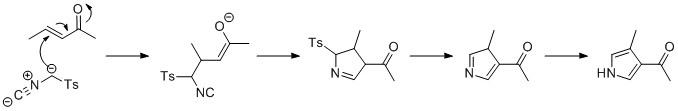 By the Barton–Zard synthesis, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-''endo''-''dig'' cyclization, elimination of the
By the Barton–Zard synthesis, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-''endo''-''dig'' cyclization, elimination of the  Pyrroles can also be prepared by
Pyrroles can also be prepared by 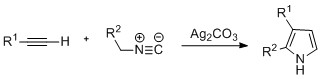 One synthetic route to pyrrole involves the
One synthetic route to pyrrole involves the  The Trofimov reaction allows for the synthesis of 2,3-disubstituted pyrroles from ketoximes and
The Trofimov reaction allows for the synthesis of 2,3-disubstituted pyrroles from ketoximes and 
 .
.
 Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
 The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
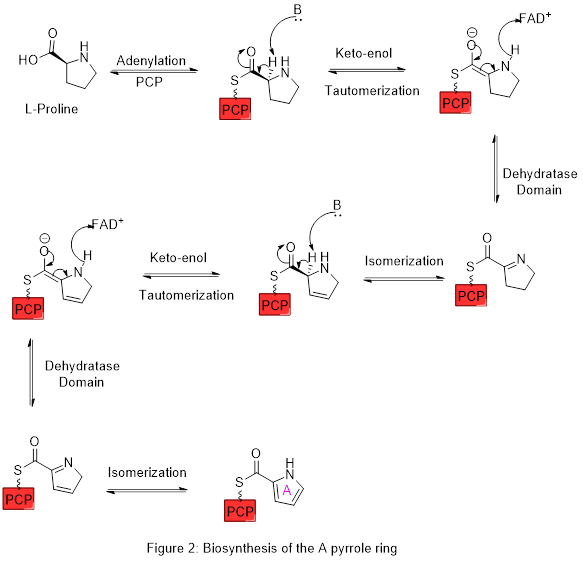
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
 Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.

 Pyrroles react easily with nitrating (e.g. HNO3/ Ac2O), sulfonating ( Py·SO3), and
Pyrroles react easily with nitrating (e.g. HNO3/ Ac2O), sulfonating ( Py·SO3), and

 Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
 Pyrroles can react with
Pyrroles can react with 
Synthesis of pyrroles (overview of recent methods)
Substitution reaction mechanisms of nitrogen-containing heteroaromatics
{{Authority control
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
, aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
, organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
, a five-membered ring
(The) Ring(s) may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
Arts, entertainment, and media Film and TV
* ''The Ring'' (franchise), a ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, . Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.
The structure of the molecule can be described ...
, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
.
Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
, the chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenation, hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine. The name chlorin derives from chlorophyll. ...
s, bacteriochlorins, and chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
s.
Properties, structure, bonding
Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified bydistillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
heterocycle, like furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
and thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has chemical shifts at 6.68 (H2, H5) and 6.22 (H3, H4). Pyrrole is an extremely weak base for an amine, with a conjugate acid p''K''a of −3.8. The most thermodynamically stable pyrrolium cation (C4H6N+) is formed by protonation at the 2 position. Substitution of pyrrole with alkyl substituents provides a more basic molecule—for example, tetramethylpyrrole has a conjugate acid p''K''a of +3.7. Pyrrole is also weakly acidic at the N–H position, with a p''K''a of 16.5.
As a hydrogen bonding Lewis acid it is classified as a hard acid and the ECW model
In chemistry, the ECW model is a semi-quantitative model that describes and predicts the strength of Lewis acid–Lewis base interactions. Many chemical reactions can be described as acid–base reactions, so models for such interactions are of pot ...
lists its acid parameters as ''E''A = 1.38 and ''C''A = 0.68.
Pyrrole has aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
character because the lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s on the nitrogen atom is partially delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
into the ring, creating a 4''n'' + 2 aromatic system (see Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
). In terms of its aromaticity, pyrrole's is modest relative to benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
but comparable to related heterocycles thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
and furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
. The resonance energies of benzene, pyrrole, thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
, and furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
are, respectively, 152, 88, 121, and 67 kJ/mol (36, 21, 29, and 16 kcal/mol). The molecule is flat.
History
Pyrrole was first detected by F. F. Runge in 1834, as a constituent ofcoal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoria ...
. In 1857, it was isolated from the pyrolysate of bone. Its name comes from the Greek ''pyrrhos'' (, "reddish, fiery"), from the reaction used to detect it—the red color that it imparts to wood when moistened with hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
.
Occurrence in nature
natural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
. Common naturally produced molecules containing pyrroles include vitamin B12, bile pigments like bilirubin
Bilirubin (BR) (adopted from German, originally bili—bile—plus ruber—red—from Latin) is a red-orange compound that occurs in the normcomponent of the straw-yellow color in urine. Another breakdown product, stercobilin, causes the brown ...
and biliverdin
Biliverdin (from the Latin for green bile) is a green tetrapyrrolic bile pigment, and is a product of heme catabolism.Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984–986. Elsevier Saunders, United States. ...
, and the porphyrins of heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
, chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
, chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenation, hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine. The name chlorin derives from chlorophyll. ...
s, bacteriochlorins, and porphyrinogens. Other pyrrole-containing secondary metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
s include PQQ, makaluvamine M, ryanodine, rhazinilam, lamellarin, prodigiosin, myrmicarin, and sceptrin. The syntheses of pyrrole-containing haemin, synthesized by Hans Fischer was recognized by the Nobel Prize.
Pyrrole is a constituent of tobacco smoke and may contribute to its toxic effects.
Synthesis
Pyrrole is prepared industrially by treatment offuran
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
with ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
in the presence of solid acid catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s, like SiO2 and Al2O3.
Pyrrole can also be formed by catalytic dehydrogenation of pyrrolidine.
Several syntheses of the pyrrole ring have been described. Three routes dominate, but many other methods exist.
Hantzsch pyrrole synthesis
The Hantzsch pyrrole synthesis is the reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles (3).Knorr pyrrole synthesis
The Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound. The method involves the reaction of an α-amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
(1) and a compound containing a methylene group α to (bonded to the next carbon to) a carbonyl group
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes ...
(2).
Paal–Knorr pyrrole synthesis
In the Paal–Knorr pyrrole synthesis, a 1,4-dicarbonyl compound reacts with ammonia or a primary amine to form a substituted pyrrole.Other methods
Van Leusen reaction pyrroles are produced by reaction oftosylmethyl isocyanide
TosMIC (toluenesulfonylmethyl isocyanide) is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functi ...
(TosMIC) with an enone in the presence of base, in a Michael addition. A 5-''endo'' cyclization then forms the 5-membered ring, which reacts to eliminate the tosyl group. The last step is tautomerization to the pyrrole.
: By the Barton–Zard synthesis, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-''endo''-''dig'' cyclization, elimination of the
By the Barton–Zard synthesis, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-''endo''-''dig'' cyclization, elimination of the nitro group
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nit ...
, and tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the reloca ...
ization.
The starting materials in the Piloty–Robinson pyrrole synthesis, named for Gertrude and Robert Robinson and Oskar Piloty, are two equivalents of an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
and hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
. The product is a pyrrole with substituents at the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
(R−C=N−N=C−R). In the second step, a ,3 sigmatropic rearrangement takes place between. Addition of hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
leads to ring closure and loss of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
to form the pyrrole. The mechanism
Mechanism may refer to:
*Mechanism (economics), a set of rules for a game designed to achieve a certain outcome
**Mechanism design, the study of such mechanisms
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a ...
was developed by the Robinsons.
In one modification, propionaldehyde
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially.
Production
Propiona ...
is treated first with hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
and then with benzoyl chloride at high temperatures and assisted by microwave irradiation:
Pyrroles bearing multiple substituents have been obtained from the reaction of münchnone
Münchnone (synonyms: 1,3-oxazolium-5-oxide; 1,3-oxazolium-5-olate; anhydro-5-hydroxy-1,3-oxazolium hydroxide; 5-hydroxy-1,3-oxazolium hydroxide, inner salt; oxido-oxazolium) is a mesoionic heterocyclic compound, heterocyclic aromatic chemical com ...
s and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s. The reaction mechanism involves 1,3-dipolar cycloaddition followed by loss of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
by a retro- Diels–Alder process. Similar reactions can be performed using azalactones.
 Pyrroles can also be prepared by
Pyrroles can also be prepared by silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
-catalyzed cyclization of alkynes with isonitriles, where R2 is an electron-withdrawing group, and R1 is an alkane, aryl group, or ester. Examples of disubstituted alkynes have also been seen to form the desired pyrrole in considerable yield. The reaction is proposed to proceed via a silver acetylide
In chemistry, an acetylide is a compound that can be viewed as the result of replacing one or both hydrogen atoms of acetylene (ethyne) by metallic or other cations. Calcium carbide is an important industrial compound, which has long been used ...
intermediate. This method is analogous to the azide–alkyne click chemistry
Click chemistry is an approach to chemical synthesis that emphasizes efficiency, simplicity, selectivity, and modularity in chemical processes used to join molecular building blocks. It includes both the development and use of "click reactions", a ...
used to form azoles.
 One synthetic route to pyrrole involves the
One synthetic route to pyrrole involves the decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
of ammonium mucate, the ammonium salt of mucic acid. The salt is typically heated in a distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
setup with glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pha ...
as a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
.
acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
in basic medium.

Biosynthesis
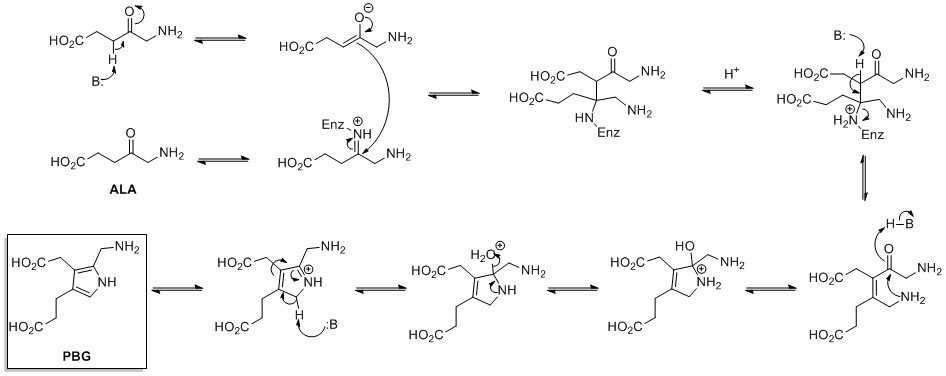
The biosynthesis of pyrrole rings begins with aminolevulinic acid (ALA), which is synthesized fromglycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
and succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A.
Sources
It is an important intermediate in the citric acid cycle, where it is synthesized from Alpha-Ketoglutaric acid, α-ketoglutarate ...
. ALA dehydratase catalyzes the condensation of two ALA molecules via a Knorr-type ring synthesis to form porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.
The structure of the molecule can be described ...
(PBG). This later reacts to form, for example, the macrocycles heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
and chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
.
 .
.
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the p ...
is biosynthetically derived from the amino acid L-glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that ...
. Glutamate-5-semialdehyde is first formed by glutamate 5-kinase (ATP-dependent) and glutamate-5-semialdehyde dehydrogenase (which requires NADH or NADPH). This can then either spontaneously cyclize to form 1-pyrroline-5-carboxylic acid, which is reduced to proline by pyrroline-5-carboxylate reductase (using NADH or NADPH), or turned into ornithine
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of th ...
by ornithine aminotransferase, followed by cyclisation by ornithine cyclodeaminase to form proline.
 Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
 The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
 Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.

Reactions and reactivity
Due to its aromatic character, pyrrole is difficult to hydrogenate, does not easily react as adiene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
in Diels–Alder reactions, and does not undergo usual olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
reactions. Its reactivity is similar to that of benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
and aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
, in that it is easy to alkylate and acylate. Under acidic conditions, pyrroles oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
easily to polypyrrole, and thus many electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
reagents that are used in benzene chemistry are not applicable to pyrroles. In contrast, substituted pyrroles (including protected pyrroles) have been used in a broad range of transformations.
Reaction of pyrrole with electrophiles
Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate. Pyrroles react easily with nitrating (e.g. HNO3/ Ac2O), sulfonating ( Py·SO3), and
Pyrroles react easily with nitrating (e.g. HNO3/ Ac2O), sulfonating ( Py·SO3), and halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
ating (e.g. NCS, NBS, Br2, SO2Cl2, and KI/ H2O2) agents. Halogenation generally provides polyhalogenated pyrroles, but monohalogenation can be performed. As is typical for electrophilic additions to pyrroles, halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen. This is a useful method for further functionalization of the generally less reactive 3-position.
Acylation
Acylation generally occurs at the 2-position, through the use of various methods. Acylation withacid anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one equivalent of wa ...
s and acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s can occur with or without a catalyst. 2-Acylpyrroles are also obtained from reaction with nitriles, by the Houben–Hoesch reaction. Pyrrole aldehydes can be formed by a Vilsmeier–Haack reaction.

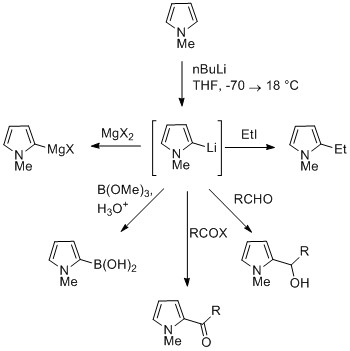
Reaction of deprotonated pyrrole
The NH proton in pyrroles is moderately acidic with a p''K''a of 17.5. Pyrrole can be deprotonated with strong bases such asbutyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
and sodium hydride. The resulting alkali pyrrolide is nucleophilic. Treating this conjugate base with an electrophile such as iodomethane gives ''N''-methylpyrrole.
''N''-Metalated pyrrole can react with electrophiles at the N or C positions, depending on the coordinating metal. More ionic nitrogen–metal bonds (such as with lithium, sodium, and potassium) and more solvating solvents lead to ''N''-alkylation. Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom. In the cases of ''N''-substituted pyrroles, metalation of the carbons is more facile. Alkyl groups can be introduced as electrophiles, or by cross-coupling reactions.
 Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
Reductions
Pyrroles can undergo reductions to pyrrolidines and to pyrrolines. For example,Birch reduction
The Birch reduction or Metal-Ammonia reduction is an organic reaction that is used to convert arenes to Cyclohexa-1,4-diene, 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch (organic chemist), Arthur Birch and i ...
of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.
Cyclization reactions
Pyrroles with ''N''-substitution can undergocycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
reactions such as +2, +2, and +1cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.
carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s, such as dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapi ...
, in a +1cycloaddition. With dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapi ...
, a dichlorocyclopropane intermediate is formed, which breaks down to form 3-chloropyridine (the Ciamician–Dennstedt rearrangement).

Commercial uses
Polypyrrole is of some commercial value. ''N''-Methylpyrrole is a precursor to ''N''-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry. Pyrroles are also found in several drugs, includingatorvastatin
Atorvastatin, sold under the brand name Lipitor among others, is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a ...
, ketorolac
Ketorolac, sold under the brand name Toradol, Acular and Sprix, among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain. Specifically it is recommended for moderate to severe pain. Recommended duration of treatment is l ...
, and sunitinib. Pyrroles are used as lightfast red, scarlet, and carmine pigments.
Analogs and derivatives
Structural analog
A structural analog, also known as a chemical analog or simply an analog, is a chemical compound, compound having a chemical structure, structure similar to that of another compound, but differing from it in respect to a certain component.
It can ...
s of pyrrole include:
* Pyrroline, a partially saturated analog with one double bond
* Pyrrolidine, the saturated hydrogenated analog
Derivatives of pyrrole include indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
, a derivative with a fused benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
ring.
See also
* Simple aromatic rings *Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( or units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon ...
* Polypyrrole
* Azonine
Azonine is an unsaturated heterocycle of nine atoms, with a nitrogen replacing a carbon at one position. A variety of derivatives have been synthesised. It is considered to possess a considerable amount of aromatic stability. It and C9H9– are ...
References
Further reading
* *External links
Synthesis of pyrroles (overview of recent methods)
Substitution reaction mechanisms of nitrogen-containing heteroaromatics
{{Authority control