Oros on:
[Wikipedia]
[Google]
[Amazon]
 The osmotic-controlled release oral delivery system (OROS) is an advanced
The osmotic-controlled release oral delivery system (OROS) is an advanced
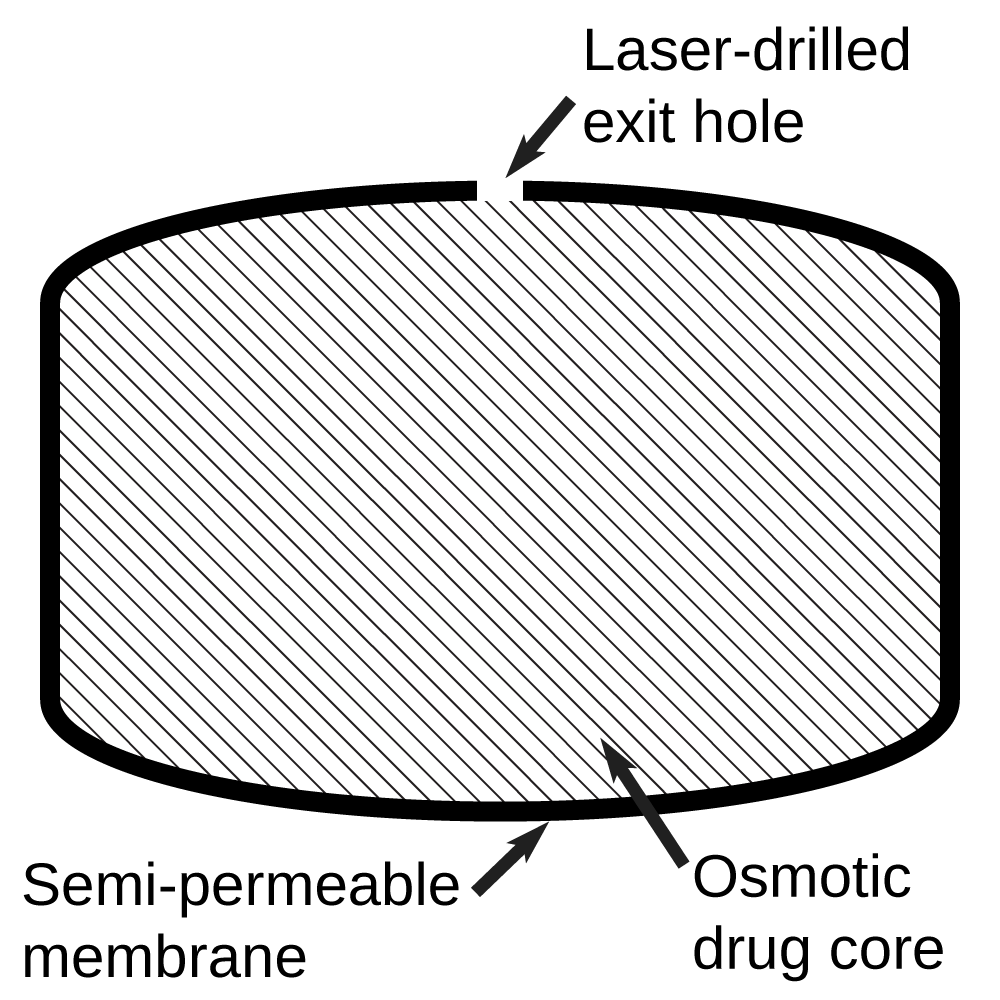 The Elementary Osmotic Pump (EOP) was developed by
The Elementary Osmotic Pump (EOP) was developed by
 Both the EOP and CPOP were relatively simple designs, and were limited by their inability to deliver poorly
Both the EOP and CPOP were relatively simple designs, and were limited by their inability to deliver poorly  In the early 1990s, an ALZA-funded research program began to develop a new dosage form of
In the early 1990s, an ALZA-funded research program began to develop a new dosage form of 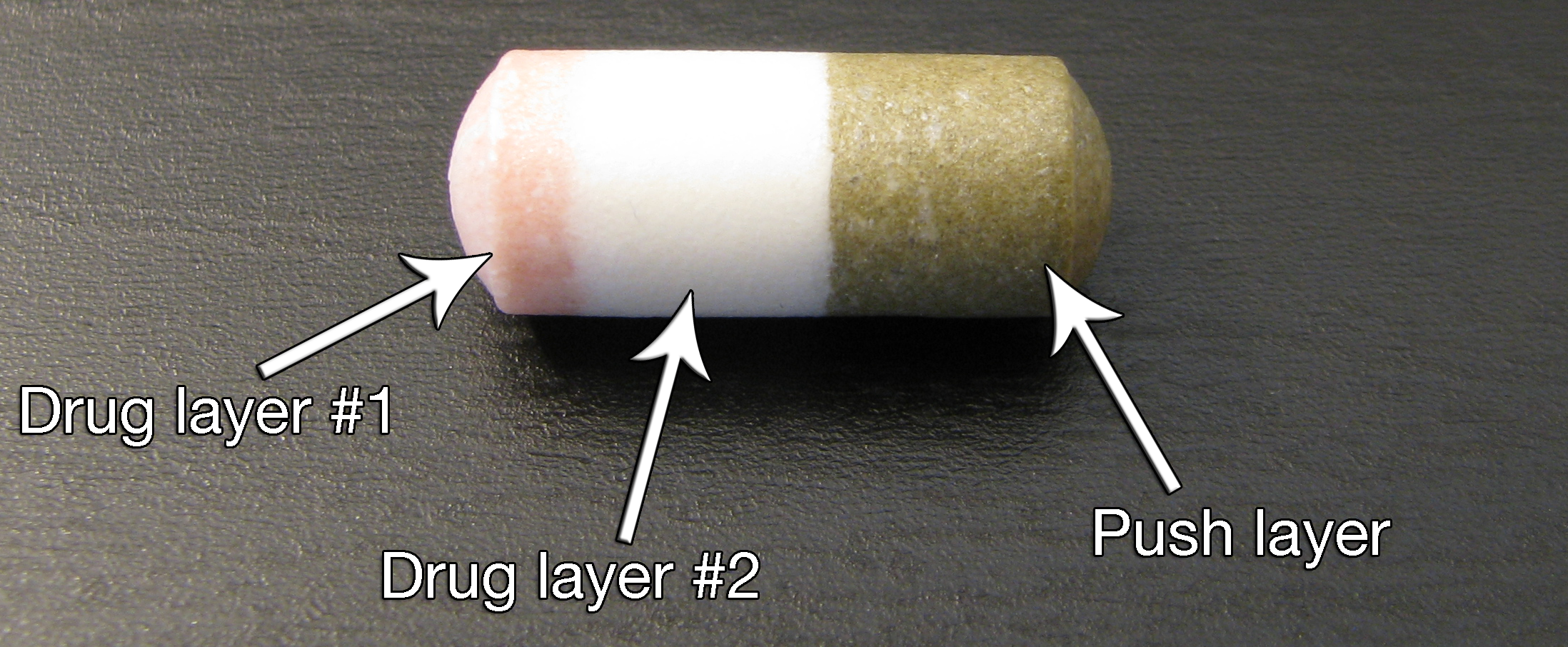
 The osmotic-controlled release oral delivery system (OROS) is an advanced
The osmotic-controlled release oral delivery system (OROS) is an advanced controlled release
Modified-release dosage is a mechanism that (in contrast to immediate-release dosage) delivers a drug with a delay after its administration (delayed-release dosage) or for a prolonged period of time (extended-release R, XR, XLdosage) or to a spe ...
oral drug delivery system in the form of a rigid tablet with a semi-permeable outer membrane and one or more small laser drilled holes in it. As the tablet passes through the body, water is absorbed through the semipermeable membrane
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecul ...
via osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of ...
, and the resulting osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a solution to take in a pure ...
is used to push the active drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insuffla ...
through the laser drilled opening(s) in the tablet and into the gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organ (biology), organs of the digestive syste ...
. OROS is a trademarked name owned by ALZA Corporation
Alza Corporation was a pharmaceutical and medical systems company.
Background
Founded in 1968 by Dr. Alejandro Zaffaroni; the company's name is a portmanteau of his name. Alza was a major pioneer in the field of drug delivery systems, bringing ...
, which pioneered the use of osmotic pumps for oral drug delivery.
Rationale
Pros and cons
Osmotic release systems have a number of major advantages over other controlled-release mechanisms. They are significantly less affected by factors such as pH, food intake, GI motility, and differing intestinal environments. Using an osmotic pump to deliver drugs has additional inherent advantages regarding control over drug delivery rates. This allows for much more precise drug delivery over an extended period of time, which results in much more predictablepharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered ...
. However, osmotic release systems are relatively complicated, somewhat difficult to manufacture, and may cause irritation or even blockage of the GI tract due to prolonged release of irritating drugs from the non-deformable tablet.
Oral osmotic release systems
Single-layer
 The Elementary Osmotic Pump (EOP) was developed by
The Elementary Osmotic Pump (EOP) was developed by ALZA
Alza Corporation was a pharmaceutical and medical systems company.
Background
Founded in 1968 by Dr. Alejandro Zaffaroni; the company's name is a portmanteau of his name. Alza was a major pioneer in the field of drug delivery systems, bringing ...
in 1974, and was the first practical example of an osmotic pump based drug release system for oral use. It was introduced to the market in the early 1980s in Osmosin (indomethacin
Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription drug, prescription medication to reduce fever, pain, joint stiffness, stiffness, and swelling (medical), swelling from infl ...
) and Acutrim (phenylpropanolamine
Phenylpropanolamine (PPA) is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to contr ...
), but unexpectedly severe issues with GI irritation and cases of GI perforation led to the withdrawal of Osmosin.
Merck & Co. later developed the Controlled-Porosity Osmotic Pump (CPOP) with the intention of addressing some of the issues that led to Osmosin's withdrawal via a new approach to the final stage of the release mechanism. Unlike the EOP, the CPOP had no pre-formed hole in the outer shell for the drug to be expelled out of. Instead, the CPOP's semipermeable membrane was designed to form numerous small pores upon contact with water through which the drug would be expelled via osmotic pressure. The pores were formed via the use of a pH insensitive leachable or dissolvable additive such as sorbitol
Sorbitol (), less commonly known as glucitol (), is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol g ...
.
Multi-layer
 Both the EOP and CPOP were relatively simple designs, and were limited by their inability to deliver poorly
Both the EOP and CPOP were relatively simple designs, and were limited by their inability to deliver poorly soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
drugs. This led to the development of an additional internal "push layer" composed of material (a swellable polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
) that would expand as it absorbed water, which then pushed the drug layer (which incorporates a viscous
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inter ...
polymer for suspension of poorly soluble drugs) out of the exit hole at a controlled rate. Osmotic agents such as sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, potassium chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt ...
, or xylitol
Xylitol is a chemical compound with the formula , or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It can be classifie ...
are added to both the drug and push layers to increase the osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a solution to take in a pure ...
. The initial design developed in 1982 by ALZA researchers was designated the Push-Pull Osmotic Pump (PPOP), and Procardia XL (nifedipine
Nifedipine (3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate), sold under the brand name Adalat and Procardia, among others, is a calcium channel blocker medication used to manage angina, high blood pressure, Ra ...
) was one of the first drugs to utilize this PPOP design.
 In the early 1990s, an ALZA-funded research program began to develop a new dosage form of
In the early 1990s, an ALZA-funded research program began to develop a new dosage form of methylphenidate
Methylphenidate, sold under the brand names Ritalin and Concerta among others, is the most widely prescribed central nervous system (CNS) stimulant medication used to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, ...
for the treatment of children with attention deficit hyperactivity disorder
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterised by excessive amounts of inattention, hyperactivity, and impulsivity that are pervasive, impairing in multiple contexts, and otherwise age-inap ...
(ADHD). Methylphenidate's short half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
required multiple doses to be administered each day to attain long-lasting coverage, which made it an ideal candidate for the OROS technology. Multiple candidate pharmacokinetic
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered ...
profiles were evaluated and tested in an attempt to determine the optimal way to deliver the drug, which was especially important given the puzzling failure of an existing extended-release formulation of methylphenidate (Ritalin SR) to act as expected. The zero-order (flat) release profile that the PPOP was optimal at delivering failed to maintain its efficacy over time, which suggested that acute tolerance to methylphenidate formed over the course of the day. This explained why Ritalin SR was inferior to twice-daily Ritalin IR, and led to the hypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous obse ...
that an ascending pattern of drug delivery was necessary to maintain clinical effect. Trials designed to test this hypothesis were successful, and ALZA subsequently developed a modified PPOP design that utilized an overcoat of methylphenidate designed to release immediately and rapidly raise serum levels, followed by 10 hours of first-order (ascending) drug delivery from the modified PPOP design. This design was called the Push-Stick Osmotic Pump (PSOP), and utilized two separate drug layers with different concentrations of methylphenidate in addition to the (now quite robust) push layer.

List of OROS medications
OROS medications include:References
{{Dosage forms, state=expanded Pharmaceutical industry Drug delivery devices Dosage forms Alza brands Pharmacology Pharmacokinetics