Manganese Sulfate on:
[Wikipedia]
[Google]
[Amazon]
Manganese(II) sulfate usually refers to the
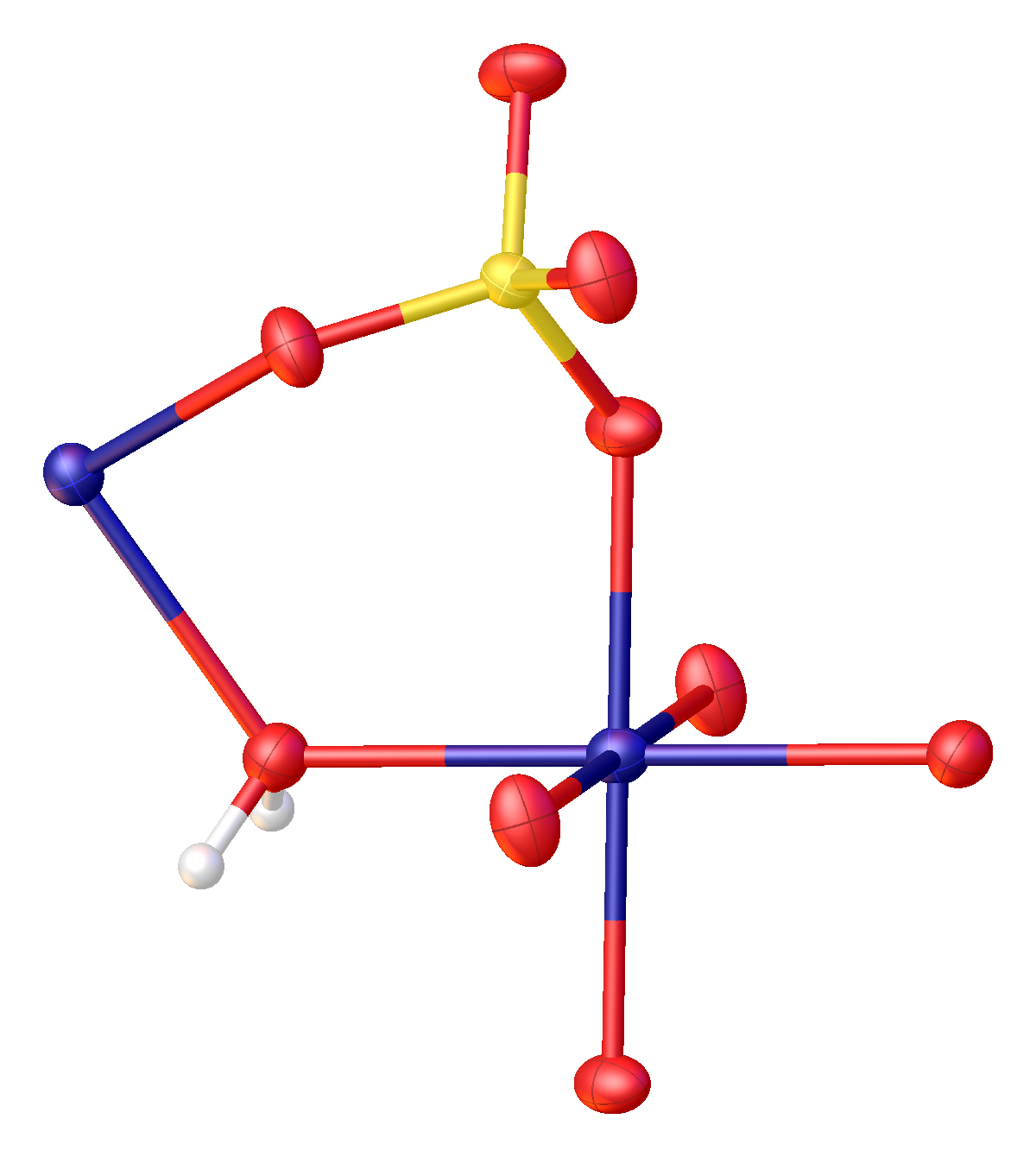 The structure of MnSO4·H2O has been determined by
The structure of MnSO4·H2O has been determined by
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
MnSO4·H2O. This pale pink deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
solid is a commercially significant manganese(II) salt. Approximately 260,000 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s of manganese(II) sulfate were produced worldwide in 2005. It is the precursor to manganese metal and many other chemical compounds
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
. Manganese-deficient soil is remediated with this salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
.
Structure
 The structure of MnSO4·H2O has been determined by
The structure of MnSO4·H2O has been determined by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. Like many metal sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
s, manganese sulfate forms a variety of Water of hydration, hydrates: monohydrate, tetrahydrate, pentahydrate, and heptahydrate. All of these salts dissolve in water to give faintly pink solutions of the Metal aquo complex, aquo complex [Mn(H2O)6]2+.
Applications and production
Typically, :Manganese minerals, manganese ores are purified by their conversion to manganese(II) sulfate. Treatment of aqueous solutions of the sulfate with sodium carbonate leads to precipitation of manganese carbonate, which can be calcined to give the oxides Manganese(II) oxide, MnO''x''. In the laboratory, manganese sulfate can be made by treating manganese dioxide with sulfur dioxide: :MnO2 + SO2 + H2O → MnSO4(H2O) It can also be made by mixing potassium permanganate with sodium bisulfate and hydrogen peroxide. Manganese sulfate is a by-product of various industrially significant oxidations that use manganese dioxide, including the manufacture of hydroquinone and anisaldehyde.Electrolysis
Electrolysis of manganese sulfate reverses the above reaction yield (chemistry), yielding manganese dioxide, which is called EMD for electrolytic manganese dioxide. Alternatively oxidation of manganese sulfate with potassium permanganate yields the so-called chemical manganese dioxide (CMD). These materials, especially EMD, are used in Dry cell battery, dry-cell batteries.Natural occurrence
Manganese(II) sulfate minerals are very rare in nature and always occur as hydrates. The monohydrate is called szmikite; tetrahydrate = ilesite; hexahydrate (the most rare) = chvaleticeite; pentahydrate = jōkokuite; heptahydrate = mallardite.References
{{Sulfates Manganese(II) compounds Sulfates Deliquescent substances