Michaelis–Arbuzov Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Michaelis–Arbuzov reaction (also called the Arbuzov reaction) is the  The reaction was discovered by
The reaction was discovered by
RCOX > RCH2X > RR'CHX \gg RR'R''CX
and
:RI > RBr > RCl
In general, tertiary alkyl halides, aryl halides and vinyl halides do not react. There are notable exceptions to this trend, including 1,2-dichloroethene and 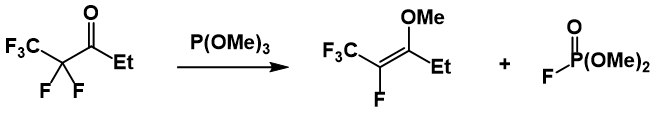
ABP-OR with A and B generally being alkyl, alkoxy or aryloxy groups. Electron-withdrawing groups are known to slow down the rate of the reaction, with electron donating groups increasing the rate of the reaction. This is consistent with initial attack of the phosphorus reagent on the alkyl halide as the  Phosphite salts (Ex: R = Na) can also undergo the reaction with precipitation of the corresponding Na-halide salt. Amidophosphites and silyloxyphosphites have been used before to yield amidophosphonates and phosphinic acids.
Phosphite salts (Ex: R = Na) can also undergo the reaction with precipitation of the corresponding Na-halide salt. Amidophosphites and silyloxyphosphites have been used before to yield amidophosphonates and phosphinic acids.
 An Arbuzov type rearrangement can also occur where the O from an OR group acts as the leaving group in the initial SN2 attack of the phosphorus. This is only known to occur when A and B are Cl.
An Arbuzov type rearrangement can also occur where the O from an OR group acts as the leaving group in the initial SN2 attack of the phosphorus. This is only known to occur when A and B are Cl.
 Phosphite esters are the least reactive class of reagents used in this reaction. They react to produce phosphonates. They require the most heating for the reaction to occur (120 °C - 160 °C is common). This high temperature allows for fractional distillation to be employed in the removal of the alkyl halide produced, though excess of the starting alkyl halide can also be used. Solvents are often not used for this reaction, though there is precedent for the improvement of selectivity with its usage.
Phosphonites are generally more reactive than phosphite esters. They react to produce phosphinates. Heating is also required for the reaction, but
Phosphite esters are the least reactive class of reagents used in this reaction. They react to produce phosphonates. They require the most heating for the reaction to occur (120 °C - 160 °C is common). This high temperature allows for fractional distillation to be employed in the removal of the alkyl halide produced, though excess of the starting alkyl halide can also be used. Solvents are often not used for this reaction, though there is precedent for the improvement of selectivity with its usage.
Phosphonites are generally more reactive than phosphite esters. They react to produce phosphinates. Heating is also required for the reaction, but
Article
* Davidsen, S. K.; Phllips, G. W.; Martin, S. F. ''Organic Syntheses'', Coll. Vol. 8, p. 451 (1993); Vol. 65, p. 119 (1987).
Article
* Enders, D.; von Berg, S.; Jandeleit, B. ''Organic Syntheses'', Coll. Vol. 10, p. 289 (2004); Vol. 78, p. 169 (2002).
Article
{{DEFAULTSORT:Michaelis-Arbuzov reaction Substitution reactions Name reactions
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
of a trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
phosphorus ester with an alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
to form a pentavalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the Arbuzov reaction; phosphite ester
file:Phosphite.svg, The general structure of a phosphite ester showing the lone pairs on the P
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be conside ...
s (1) react to form phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing Functional group, groups, where R is an organic group (alkyl, aryl). If R is hydrogen then the compound is a Phosphite_ester#Chemistry_of_HP(O)(OR ...
s (2), phosphonites (3) react to form phosphinates (4) and phosphinites (5) react to form phosphine oxide
Phosphine oxide is the inorganic compound with the formula H3PO. Although stable as a dilute gas, liquid or solid samples are unstable. Unlike many other compounds of the type POxHy, H3PO is rarely discussed and is not even mentioned in major so ...
s (6).
 The reaction was discovered by
The reaction was discovered by August Michaelis
August Michaelis (26 December 1847 – 31 January 1916) was a German people, German chemist and discovered the Michaelis–Arbuzov reaction.
Michaelis studied at the University of Göttingen and University of Jena and became professor for chemist ...
in 1898, and greatly explored by Aleksandr Arbuzov soon thereafter. This reaction is widely used for the synthesis of various phosphonates, phosphinates, and phosphine oxide
Phosphine oxide is the inorganic compound with the formula H3PO. Although stable as a dilute gas, liquid or solid samples are unstable. Unlike many other compounds of the type POxHy, H3PO is rarely discussed and is not even mentioned in major so ...
s. Several reviews have been published. The reaction also occurs for coordinated phosphite ligands, as illustrated by the demethylation of 2+ to give −, which is called the Klaui ligand.
Reaction mechanism
center, 600px, The mechanism of the Michaelis–Arbuzov reaction The Michaelis–Arbuzov reaction is initiated with the SN2 attack of the nucleophilic phosphorus species (1 - A phosphite) with theelectrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
alkyl halide (2) to give a phosphonium salt
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The salt ...
as an intermediate (3). These intermediates are occasionally stable enough to be isolated, such as for triaryl phosphites which do not react to form the phosphonate without thermal cleavage of the intermediate (200 °C), or cleavage by alcohols or bases. The displaced halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
anion then usually reacts via another SN2 reaction on one of the R1 carbons, displacing the oxygen atom to give the desired phosphonate (4) and another alkyl halide (5). This has been supported by the observation that chiral R1 groups experience inversion of configuration at the carbon center attacked by the halide anion. This is what is expected of an SN2 reaction. Evidence also exists for a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
based mechanism of dealkylation similar to an SN1 reaction, where the R1 group initially dissociates from the phosphonium salt followed by attack of the anion. Phosphite esters with tertiary alkyl halide groups can undergo the reaction, which would be unexpected if only an SN2 mechanism was operating. Further support for this SN1 type mechanism comes from the use of the Arbuzov reaction in the synthesis of neopentyl halides, a class of compounds that are notoriously unreactive towards SN2 reactions. Based on the principle of microscopic reversibility The principle of microscopic reversibility in physics and chemistry is twofold:
* First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with respe ...
, the inert nature of the neopentyl halides towards the SN2 reaction indicates that an SN2 reaction is unlikely to be the mechanism for the synthesis of the neopentyl halides in this reaction. Substrates that cannot react through an SN2 pathway or an SN1 pathway generally do not react, which include vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
groups. For example, the triaryl phosphites mentioned above generally do not react because they form stable phosphonium salts. Since aryl groups do not undergo SN1 and SN2 type mechanisms, triaryl phosphites lack a low energy pathway for decomposition of the phosphonium salt. An allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction, organic chemical reaction in which reaction at a center Vicinal (chemistry), vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms:
It is encountered ...
mechanism (SN2') has also been implicated in allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
and propargyl
In organic chemistry, the propargyl group is a functional group of 2- propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework ...
halides.
Stereochemical experiments on cyclic phosphites have revealed the presence of both pentavalent phosphoranes and tetravalent phosphonium intermediates in chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
being involved in the dealkylation step of the reaction using 31P NMR. The decomposition of these intermediates is driven primarily by the nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
of the anion. There exists many instances of the intermediate phosphonium salts being sufficiently stable that they can be isolated when the anion is weakly nucleophilic, such as with tetrafluoroborate or triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
anions.
Scope
Alkyl halide
Source: As a general guideline, the reactivity of the organic halide component can be listed as follows: (from most reactive to least reactive) :trityl
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the chemical formula, formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic sk ...
halides. Some activated aryl halides, often involving heterocycles have been known to undergo the reaction. Iodobenzene
Iodobenzene is an aryl iodide and the simplest of the iodobenzenes, consisting of a benzene ring substituted with one iodine atom. Its chemical formula is . It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorles ...
and substituted derivatives have been known to undergo the reaction under photolytic conditions. Secondary alkyl halides often do not react well, producing alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s as side-products. Allyl and propargyl halides are also reactive, but can proceed through an SN2 or an SN2` mechanism. Reaction with primary alkyl halides and acyl halides generally proceed smoothly. Carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
interestingly enough, only undergoes the reaction a single time with chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
being inert to the reaction conditions. When a halide atom is found in the ester chain off of the phosphorus atom, isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
to the corresponding Arbuzov product has been known without addition of an alkyl halide.
The Perkow reaction
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl group, vinyl phosphate and an alkyl halide.
In the related Michaelis–Arbuzov reaction the same reactants are known ...
is a competing reaction pathway for α-bromo- and α-chloroketones. Under the reaction conditions a mixture of the Perkow product and the normal Arbuzov product occur, usually favoring the Perkow product by a significant amount. Using higher temperatures during the reaction can lead to favoring of the Arbuzov product. The reaction of α-iodoketones give only the Arbuzov product. Other methods of producing β-ketophosphonates have been developed.
The reaction of trivalent phosphorus compounds with alkyl fluorides is abnormal. One example of this reactivity is shown below.

Phosphorus reactant
The general form of the trivalent phosphorus reagent can be considered as follows:rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
of the reaction. The reaction proceeds smoothly when the R group is aliphatic. When all of A, B and R are aryl groups, a stable phosphonium salt is formed and the reaction proceeds no further under normal conditions. Heating to higher temperatures in the presence of alcohols has been known to give the isomerization product. Cyclic phosphites generally react to eject the non-cyclic OR group, though for some 5-member rings additional heating is required to afford the final cyclic product.
 Phosphite salts (Ex: R = Na) can also undergo the reaction with precipitation of the corresponding Na-halide salt. Amidophosphites and silyloxyphosphites have been used before to yield amidophosphonates and phosphinic acids.
Phosphite salts (Ex: R = Na) can also undergo the reaction with precipitation of the corresponding Na-halide salt. Amidophosphites and silyloxyphosphites have been used before to yield amidophosphonates and phosphinic acids.
 An Arbuzov type rearrangement can also occur where the O from an OR group acts as the leaving group in the initial SN2 attack of the phosphorus. This is only known to occur when A and B are Cl.
An Arbuzov type rearrangement can also occur where the O from an OR group acts as the leaving group in the initial SN2 attack of the phosphorus. This is only known to occur when A and B are Cl.
pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of the ester to an acid is a common side reaction. The poor availability of substituted phosphonites limits the usage of this class of reagent in the Arbuzov reaction. Hydroxy, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
, carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
, primary and secondary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
functional groups cannot be used with phosphonites in the reaction as they all react with the phosphonite.
Phosphinites are the most reactive class of reagents used in this reaction. They react to produce phosphine oxides. They often require very little heating (45 °C) for the reaction to occur and have been known to self-isomerize without the presence of alkyl halides.
The Arbuzov rearrangement generally does not admit a thiologous analogue, except when the phosphorus is substituted with strongly electron-donating groups. although note that Almasi identifies the chemoselective axis as hard-softness instead.
See also
* Abramov reaction *Perkow reaction
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl group, vinyl phosphate and an alkyl halide.
In the related Michaelis–Arbuzov reaction the same reactants are known ...
* Michaelis–Becker reaction
* Hirao coupling
References
External links
* Ford-Moore, A. H.; Perry, B. J. ''Organic Syntheses'', Coll. Vol. 4, p. 325 (1963); Vol. 31, p. 33 (1951).Article
* Davidsen, S. K.; Phllips, G. W.; Martin, S. F. ''Organic Syntheses'', Coll. Vol. 8, p. 451 (1993); Vol. 65, p. 119 (1987).
Article
* Enders, D.; von Berg, S.; Jandeleit, B. ''Organic Syntheses'', Coll. Vol. 10, p. 289 (2004); Vol. 78, p. 169 (2002).
Article
{{DEFAULTSORT:Michaelis-Arbuzov reaction Substitution reactions Name reactions