Kumada–Tamao–Corriu Coupling on:
[Wikipedia]
[Google]
[Amazon]
 In
In

 Current understanding of the mechanism for the nickel-catalyzed coupling is limited. Indeed, the reaction mechanism is believed to proceed differently under different reaction conditions and when using different nickel ligands. In general the mechanism can still be described as analogous to the palladium scheme (right). Under certain reaction conditions, however, the mechanism fails to explain all observations. Examination by Vicic and coworkers using tridentate terpyridine ligand identified intermediates of a Ni(II)-Ni(I)-Ni(III) catalytic cycle, suggesting a more complicated scheme. Additionally, with the addition of butadiene, the reaction is believed to involve a Ni(IV) intermediate.
Current understanding of the mechanism for the nickel-catalyzed coupling is limited. Indeed, the reaction mechanism is believed to proceed differently under different reaction conditions and when using different nickel ligands. In general the mechanism can still be described as analogous to the palladium scheme (right). Under certain reaction conditions, however, the mechanism fails to explain all observations. Examination by Vicic and coworkers using tridentate terpyridine ligand identified intermediates of a Ni(II)-Ni(I)-Ni(III) catalytic cycle, suggesting a more complicated scheme. Additionally, with the addition of butadiene, the reaction is believed to involve a Ni(IV) intermediate.
 Though poorly understood, the mechanism of this reaction is proposed to involve the formation of an octadienyl nickel complex. This catalyst is proposed to undergo transmetalation with a Grignard reagent first, prior to the reductive elimination of the halide, reducing the risk of β-hydride elimination. However, the presence of a Ni(IV) intermediate is contrary to mechanisms proposed for aryl or vinyl halide couplings.
Though poorly understood, the mechanism of this reaction is proposed to involve the formation of an octadienyl nickel complex. This catalyst is proposed to undergo transmetalation with a Grignard reagent first, prior to the reductive elimination of the halide, reducing the risk of β-hydride elimination. However, the presence of a Ni(IV) intermediate is contrary to mechanisms proposed for aryl or vinyl halide couplings.

website
 Conversely, a Kumada coupling using vinylic Grignard reagents proceeds without stereospecificity to form a mixture of ''cis-'' and ''trans-''alkenes. The degree of isomerization is dependent on a variety of factors including reagent ratios and the identity of the halide group. According to Kumada, this loss of stereochemistry is attributable to side-reactions between two equivalents of the allylic Grignard reagent.
Conversely, a Kumada coupling using vinylic Grignard reagents proceeds without stereospecificity to form a mixture of ''cis-'' and ''trans-''alkenes. The degree of isomerization is dependent on a variety of factors including reagent ratios and the identity of the halide group. According to Kumada, this loss of stereochemistry is attributable to side-reactions between two equivalents of the allylic Grignard reagent.

 Asymmetric Kumada couplings can be effected through the use of
Asymmetric Kumada couplings can be effected through the use of 


 Since this initial preparation, the synthesis has been improved to obtain higher yields and operate at room temperature.
Since this initial preparation, the synthesis has been improved to obtain higher yields and operate at room temperature.
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the Kumada coupling is a type of cross coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reac ...
, useful for generating carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between on ...
s by the reaction of a Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
and an organic halide. The procedure uses transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
, typically nickel or palladium, to couple a combination of two alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
or vinyl group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound contai ...
s. The groups of Robert Corriu and Makoto Kumada
was a Japanese chemist and was a Professor of Chemistry first at Osaka City University until his retirement in 1983 at Kyoto University in Japan. In 1972, Kumada's group reported nickel-catalyzed cross coupling reactions nearly concurrently wit ...
reported the reaction independently in 1972.
The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki
is a Japanese multinational mobility manufacturer headquartered in Hamamatsu, Shizuoka Prefecture, Shizuoka. It manufactures automobiles, motorcycles, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs and a va ...
, Sonogashira, Stille, Hiyama, Negishi), the Kumada coupling continues to be employed in many synthetic
Synthetic may refer to:
Science
* Synthetic biology
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic elements, chemical elements that are not naturally found on Earth and therefore have to be created in ...
applications, including the industrial-scale production of aliskiren
Aliskiren (brand names Tekturna and Rasilez) is the first in a class of drugs called direct renin inhibitors. It is used for essential (primary) hypertension. While used for high blood pressure, other better studied medications are typically rec ...
, a hypertension
Hypertension, also known as high blood pressure, is a Chronic condition, long-term Disease, medical condition in which the blood pressure in the artery, arteries is persistently elevated. High blood pressure usually does not cause symptoms i ...
medication, and polythiophenes, useful in organic electronic devices.
History
The first investigations into the catalytic coupling of Grignard reagents with organic halides date back to the 1941 study ofcobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
catalysts by Morris S. Kharasch
Morris Selig Kharasch (August 24, 1895 – October 9, 1957) was a pioneering organic chemist best known for his work with free radical additions and polymerizations. He defined the peroxide effect, explaining how an anti-Markovnikov orientation c ...
and E. K. Fields. In 1971, Tamura and Kochi elaborated on this work in a series of publications demonstrating the viability of catalysts based on silver, copper and iron. However, these early approaches produced poor yields due to substantial formation of homocoupling products, where two identical species are coupled.
These efforts culminated in 1972, when the Corriu and Kumada groups concurrently reported the use of nickel-containing catalysts. With the introduction of palladium catalysts in 1975 by the Murahashi group, the scope of the reaction was further broadened. Subsequently, many additional coupling techniques have been developed, culminating in the 2010 Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
recognized Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negi ...
, Akira Suzuki
is a Japanese chemist and Nobel Prize Laureate (2010), who first published the Suzuki reaction, the organic reaction of an aryl- or vinyl- boronic acid with an aryl- or vinyl- halide catalyzed by a palladium(0) complex, in 1979.
Early life a ...
and Richard F. Heck
Richard Frederick Heck (August 15, 1931 – October 9, 2015) was an American chemist noted for the discovery and development of the Heck reaction, which uses palladium to catalyze organic chemical reactions that couple aryl halides with alken ...
for their contributions to the field.
Mechanism

Palladium catalysis
According to the widely accepted mechanism, the palladium-catalyzed Kumada coupling is understood to be analogous to palladium's role in other cross coupling reactions. The proposed catalytic cycle involves both palladium(0) and palladium(II) oxidation states. Initially, the electron-rich Pd(0) catalyst (1) inserts into the R–X bond of the organic halide. Thisoxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
forms an organo-Pd(II)-complex (2). Subsequent transmetalation with the Grignard reagent forms a hetero-organometallic complex (3). Before the next step, isomerization is necessary to bring the organic ligands next to each other into mutually cis positions. Finally, reductive elimination of (4) forms a carbon–carbon bond and releases the cross coupled product while regenerating the Pd(0) catalyst (1). For palladium catalysts, the frequently rate-determining oxidative addition occurs more slowly than with nickel catalyst systems.
Nickel catalysis
 Current understanding of the mechanism for the nickel-catalyzed coupling is limited. Indeed, the reaction mechanism is believed to proceed differently under different reaction conditions and when using different nickel ligands. In general the mechanism can still be described as analogous to the palladium scheme (right). Under certain reaction conditions, however, the mechanism fails to explain all observations. Examination by Vicic and coworkers using tridentate terpyridine ligand identified intermediates of a Ni(II)-Ni(I)-Ni(III) catalytic cycle, suggesting a more complicated scheme. Additionally, with the addition of butadiene, the reaction is believed to involve a Ni(IV) intermediate.
Current understanding of the mechanism for the nickel-catalyzed coupling is limited. Indeed, the reaction mechanism is believed to proceed differently under different reaction conditions and when using different nickel ligands. In general the mechanism can still be described as analogous to the palladium scheme (right). Under certain reaction conditions, however, the mechanism fails to explain all observations. Examination by Vicic and coworkers using tridentate terpyridine ligand identified intermediates of a Ni(II)-Ni(I)-Ni(III) catalytic cycle, suggesting a more complicated scheme. Additionally, with the addition of butadiene, the reaction is believed to involve a Ni(IV) intermediate.
Scope
Organic halides and pseudohalides
The Kumada coupling has been successfully demonstrated for a variety of aryl or vinyl halides. In place of the halide reagent pseudohalides can also be used, and the coupling has been shown to be quite effective usingtosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
and triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
species in variety of conditions.
Despite broad success with aryl and vinyl couplings, the use of alkyl halides is less general due to several complicating factors. Having no π-electrons, alkyl halides require different oxidative addition mechanisms than aryl or vinyl groups, and these processes are currently poorly understood. Additionally, the presence of β-hydrogens makes alkyl halides susceptible to competitive elimination processes.
These issues have been circumvented by the presence of an activating group, such as the carbonyl in α-bromoketones, that drives the reaction forward. However, Kumada couplings have also been performed with non-activated alkyl chains, often through the use of additional catalysts or reagents. For instance, with the addition of 1,3-butadienes Kambe and coworkers demonstrated nickel catalyzed alkyl–alkyl couplings that would otherwise be unreactive.
 Though poorly understood, the mechanism of this reaction is proposed to involve the formation of an octadienyl nickel complex. This catalyst is proposed to undergo transmetalation with a Grignard reagent first, prior to the reductive elimination of the halide, reducing the risk of β-hydride elimination. However, the presence of a Ni(IV) intermediate is contrary to mechanisms proposed for aryl or vinyl halide couplings.
Though poorly understood, the mechanism of this reaction is proposed to involve the formation of an octadienyl nickel complex. This catalyst is proposed to undergo transmetalation with a Grignard reagent first, prior to the reductive elimination of the halide, reducing the risk of β-hydride elimination. However, the presence of a Ni(IV) intermediate is contrary to mechanisms proposed for aryl or vinyl halide couplings.

Grignard reagent
Couplings involving aryl and vinyl Grignard reagents were reported in the original publications by Kumada and Corriu. Alkyl Grignard reagents can also be used without difficulty, as they do not suffer from β-hydride elimination processes. Although the Grignard reagent inherently has poor functional group tolerance, low-temperature syntheses have been prepared with highly functionalized aryl groups.Catalysts
Kumada couplings can be performed with a variety of nickel(II) or palladium(II) catalysts. The structures of the catalytic precursors can be generally formulated as ML2X2, where L is a phosphine ligand. Common choices for L2 include bidentate diphosphine ligands such asdppe DPPE may refer to the chemicals:
* 1,2-Bis(diphenylphosphino)ethane
* Dipalmitoylphosphatidylethanolamine
{{disambiguation ...
and dppp among others.
Work by Alois Fürstner and coworkers on iron-based catalysts have shown reasonable yields. The catalytic species in these reactions is proposed to be an "inorganic Grignard reagent" consisting of .
Reaction conditions
The reaction typically is carried out intetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
or diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
as solvent. Such ethereal solvents are convenient because these are typical solvents for generating the Grignard reagent. Due to the high reactivity of the Grignard reagent, Kumada couplings have limited functional group tolerance which can be problematic in large syntheses. In particular, Grignard reagents are sensitive to protonolysis from even mildly acidic groups such as alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
. They also add to carbonyls and other oxidative groups.
As in many coupling reactions, the transition metal palladium catalyst is often air-sensitive, requiring an inert Argon or nitrogen reaction environment.
A sample synthetic preparation is available at the ''Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
'website
Selectivity
Stereoselectivity
Both ''cis-'' and ''trans-''olefin halides promote the overall retention of geometric configuration when coupled with alkyl Grignards. This observation is independent of other factors, including the choice of catalyst ligands and vinylic substituents. Conversely, a Kumada coupling using vinylic Grignard reagents proceeds without stereospecificity to form a mixture of ''cis-'' and ''trans-''alkenes. The degree of isomerization is dependent on a variety of factors including reagent ratios and the identity of the halide group. According to Kumada, this loss of stereochemistry is attributable to side-reactions between two equivalents of the allylic Grignard reagent.
Conversely, a Kumada coupling using vinylic Grignard reagents proceeds without stereospecificity to form a mixture of ''cis-'' and ''trans-''alkenes. The degree of isomerization is dependent on a variety of factors including reagent ratios and the identity of the halide group. According to Kumada, this loss of stereochemistry is attributable to side-reactions between two equivalents of the allylic Grignard reagent.

Enantioselectivity
 Asymmetric Kumada couplings can be effected through the use of
Asymmetric Kumada couplings can be effected through the use of chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
ligands. Using planar chiral ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an o ...
ligands, enantiomeric excesses (ee) upward of 95% have been observed in aryl couplings. More recently, Gregory Fu
Gregory C. Fu is an American chemist who is a professor of organic chemistry at the California Institute of Technology, where he is the Norman Chandler Professor of Chemistry. The current research interests of the Fu laboratory include metal-cata ...
and co-workers have demonstrated enantioconvergent couplings of α-bromoketones using catalysts based on bis-oxazoline ligands, wherein the chiral catalyst converts a racemic mixture of starting material to one enantiomer of product with up to 95% ee. The latter reaction is also significant for involving a traditionally inaccessible alkyl halide coupling.

Chemoselectivity
Grignard reagents do not typically couple with chlorinated arenes. This low reactivity is the basis for chemoselectivity for nickel insertion into the C–Br bond of bromochlorobenzene using a NiCl2-based catalyst.
Applications
Synthesis of aliskiren
The Kumada coupling is suitable for large-scale, industrial processes, such as drug synthesis. The reaction is used to construct the carbon skeleton ofaliskiren
Aliskiren (brand names Tekturna and Rasilez) is the first in a class of drugs called direct renin inhibitors. It is used for essential (primary) hypertension. While used for high blood pressure, other better studied medications are typically rec ...
(trade name Tekturna), a treatment for hypertension
Hypertension, also known as high blood pressure, is a Chronic condition, long-term Disease, medical condition in which the blood pressure in the artery, arteries is persistently elevated. High blood pressure usually does not cause symptoms i ...
.

Synthesis of polythiophenes
The Kumada coupling also shows promise in the synthesis ofconjugated polymers
In physical organic chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represent ...
, polymers such as polyalkylthiophenes (PAT), which have a variety of potential applications in organic solar cells and light-emitting diodes
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresp ...
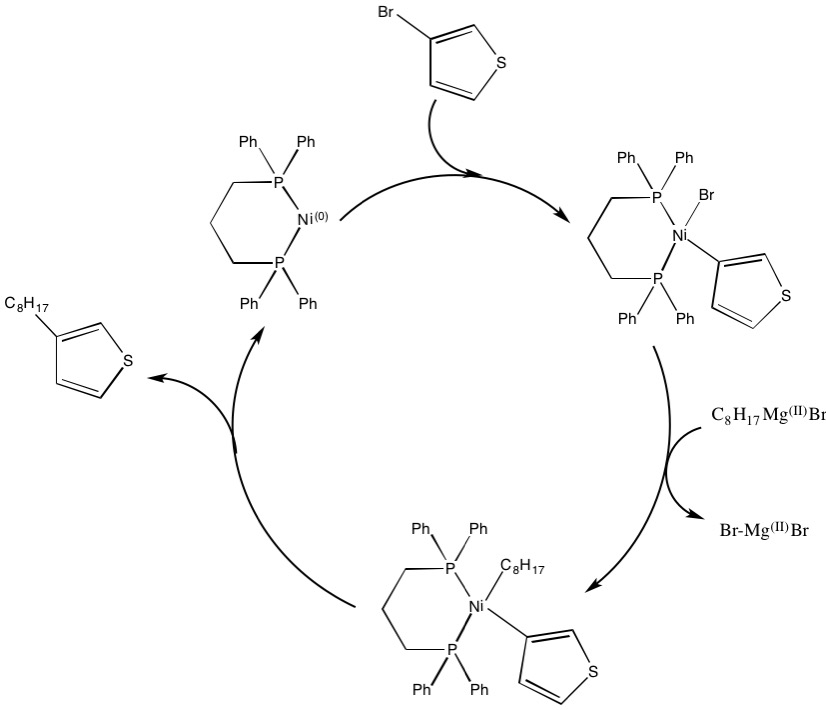
. In 1992, McCollough and Lowe developed the first synthesis of regioregular polyalkylthiophenes by utilizing the Kumada coupling scheme pictured below, which requires subzero temperatures.
 Since this initial preparation, the synthesis has been improved to obtain higher yields and operate at room temperature.
Since this initial preparation, the synthesis has been improved to obtain higher yields and operate at room temperature.
See also
*Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after T ...
* Hiyama coupling
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds (C-C bonds). This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Ha ...
* Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
* Negishi coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon–carbon bonds (C–C) in the process. A palladium (0) s ...
* Petasis reaction
The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl- boronic acid to form substituted amines.
Reported in 1993 by Nicos Petasis ...
* Stille reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin chemistry, organotin compound (also known as organostannanes). A variet ...
* Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
* Murahashi coupling
Citations
{{reflist, 30em Carbon-carbon bond forming reactions Name reactions