Intramolecular force on:
[Wikipedia]
[Google]
[Amazon]
An intramolecular force (from Latin ''intra-'' 'within') is any
 An
An
 In a true
In a true

 Bonds are formed by atoms so that they are able to achieve a lower energy state. Free atoms will have more energy than a bonded atom. This is because some energy is released during bond formation, allowing the entire system to achieve a lower energy state. The bond length, or the minimum separating distance between two atoms participating in bond formation, is determined by their repulsive and attractive forces along the internuclear direction. As the two atoms get closer and closer, the positively charged nuclei repel, creating a force that attempts to push the atoms apart. As the two atoms get further apart, attractive forces work to pull them back together. Thus an equilibrium bond length is achieved and is a good measure of bond stability.
Bonds are formed by atoms so that they are able to achieve a lower energy state. Free atoms will have more energy than a bonded atom. This is because some energy is released during bond formation, allowing the entire system to achieve a lower energy state. The bond length, or the minimum separating distance between two atoms participating in bond formation, is determined by their repulsive and attractive forces along the internuclear direction. As the two atoms get closer and closer, the positively charged nuclei repel, creating a force that attempts to push the atoms apart. As the two atoms get further apart, attractive forces work to pull them back together. Thus an equilibrium bond length is achieved and is a good measure of bond stability.
 Intramolecular forces are extremely important in the field of biochemistry, where it comes into play at the most basic levels of biological structures. Intramolecular forces such as
Intramolecular forces are extremely important in the field of biochemistry, where it comes into play at the most basic levels of biological structures. Intramolecular forces such as
force
In physics, a force is an influence that can cause an Physical object, object to change its velocity unless counterbalanced by other forces. In mechanics, force makes ideas like 'pushing' or 'pulling' mathematically precise. Because the Magnitu ...
that binds together the atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s making up a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
. Intramolecular forces are stronger than the intermolecular forces
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
that govern the interactions between molecules.
Types
The classical model identifies three main types of chemical bonds — ionic, covalent, and metallic — distinguished by the degree of charge separation between participating atoms. The characteristics of the bond formed can be predicted by the properties of constituent atoms, namely electronegativity. They differ in the magnitude of their bond enthalpies, a measure of bond strength, and thus affect the physical and chemical properties of compounds in different ways. % of ionic character is directly proportional difference in electronegativity of bonded atom.Ionic bond
 An
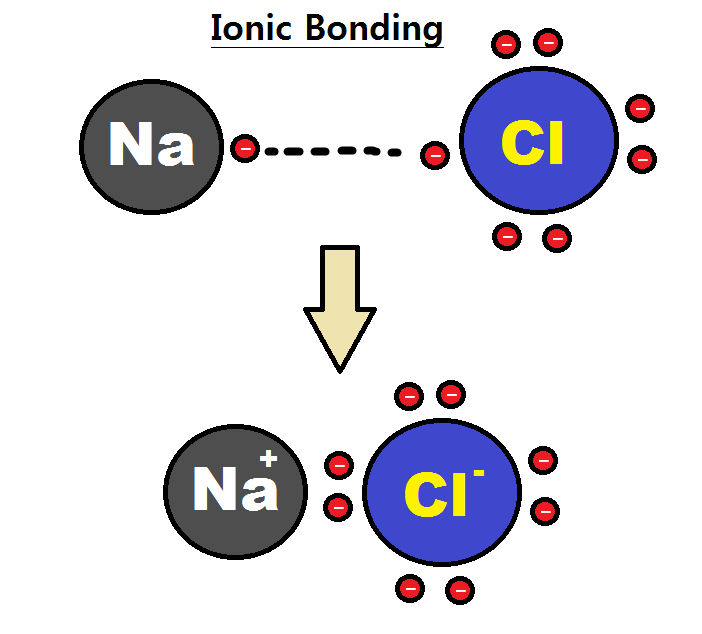
An ionic bond
Ionic bonding is a type of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic ...
can be approximated as complete transfer of one or more valence electrons of atoms participating in bond formation, resulting in a positive ion and a negative ion bound together by electrostatic forces. Electrons in an ionic bond tend to be mostly found around one of the two constituent atoms due to the large electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
difference between the two atoms, generally more than 1.9, (greater difference in electronegativity results in a stronger bond); this is often described as one atom donating electrons to the other. This type of bond is generally formed between a metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
and nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
, such as sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
and chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
in NaCl
Sodium chloride , commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral hali ...
. Sodium would give an electron to chlorine, forming a positively charged sodium ion and a negatively charged chloride ion.
Covalent bond
 In a true
In a true covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
, the electrons are uniformly shared between the two atoms of the bond; there is little or no charge separation. Covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s are generally formed between two nonmetals. There are several types of covalent bonds: in polar covalent bonds, electrons are more likely to be found around one of the two atoms, whereas in nonpolar covalent bonds, electrons are evenly shared. Homonuclear
In chemistry, homonuclear molecules, or elemental molecules, or homonuclear species, are molecules composed of only one element. Homonuclear molecules may consist of various numbers of atoms. The size of the molecule an element can form depends ...
diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
s are purely covalent. The polarity of a covalent bond is determined by the electronegativities of each atom and thus a polar covalent bond has a dipole moment pointing from the partial positive end to the partial negative end. Polar covalent bonds represent an intermediate type in which the electrons are neither completely transferred from one atom to another nor evenly shared.
Metallic bond
Metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be descr ...
s generally form within a pure metal or metal alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
. Metallic electrons are generally delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
; the result is a large number of free electrons around positive nuclei, sometimes called an electron sea.
Bond formation
Comparison of the bond lengths between carbon and oxygen in a double and triple bond.
Biochemistry
disulfide bonds
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In in ...
give proteins and DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
their structure. Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s derive their structure from the intramolecular forces that shape them and hold them together. The main source of structure in these molecules is the interaction between the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
residues that form the foundation of proteins. The interactions between residues of the same proteins forms the secondary structure of the protein, allowing for the formation of beta sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gene ...
s and alpha helices
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of l ...
, which are important structures for proteins and in the case of alpha helices, for DNA.
See also
*Chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
*Intermolecular force
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
References
{{DEFAULTSORT:Intramolecular Force Chemical bonding