IBX on:
[Wikipedia]
[Google]
[Amazon]
2-Iodoxybenzoic acid (IBX) is an
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
used in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
as an oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
. This periodinane Periodinanes also known as lambda, λ5-iodanes are organoiodine compounds with iodine in the +5 oxidation state. These compounds are described as hypervalency, hypervalent because the iodine center has more than 8 valence electrons.
Periodinane com ...
is especially suited to oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s to aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant such as potassium bromate
Potassium bromate () is a bromate of potassium and takes the form of white crystals or powder. It is a strong oxidizing agent.
Preparation and structure
Potassium bromate is produced when bromine is passed through a hot solution of potassium hyd ...
and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, or more commonly, oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. IBX is an impact- and heat-sensitive explosive (>200°C). Commercial IBX is stabilized by carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s such as benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
and isophthalic acid
Isophthalic acid is an organic compound with the formula C6H4(CO2H)2. This colorless solid is an isomer of phthalic acid and terephthalic acid. The main industrial uses of purified isophthalic acid (PIA) are for the production of polyethylene ...
.
Preparation
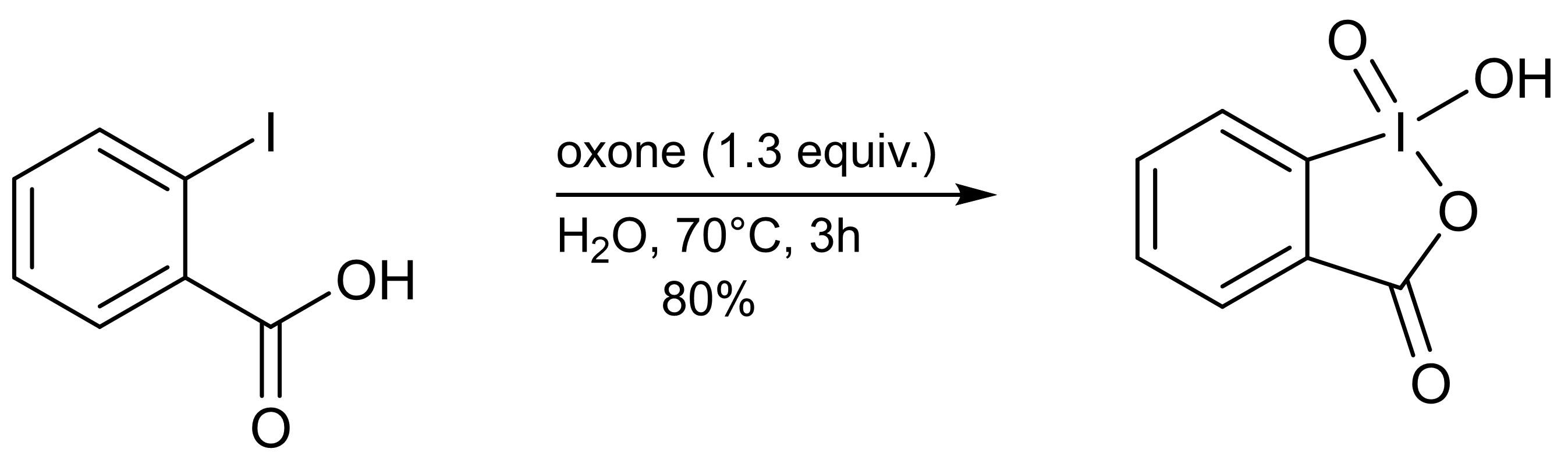
IBX can be prepared in a single step by adding an excess ofoxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
to an aqueous solution of 2-iodobenzoic acid. After warming the solution to 70°C for three hours, the precipitated IBX is collected as a white crystalline solid (80% yield, ≥95% purity). Decomposition of IBX to 2-iodosobenzoic acid and 2-iodobenzoic acid occurs at elevated temperatures, and therefore purification by recrystallization from water is not possible. Purity can be increased (≥99%) by shorting the reaction time to one hour at 70°C, at the cost of slightly reducing yield to 77%.

Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
for an oxidation of an alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
to an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
according to the hypervalent twisting mechanism involves a ligand exchange reaction replacing the hydroxyl group by the alcohol followed by a twist and an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
. The twist is a requirement because the iodine to oxygen double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
is oriented out of plane with the alkoxy
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the ...
group and the concerted elimination would not be able to take place. This twist reaction is a rearrangement in which the oxygen atom is moved into a proper plane for a 5 membered cyclic transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
in the elimination reaction and is calculated by Computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
to be the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
in the oxidation. The twist mechanism also explains why oxidation is faster for larger alcohols than for small alcohols. The twist is driven forward by the steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
that exists between the ortho hydrogen atom and the protons from the alkoxy
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the ...
group and larger alkoxy groups create larger steric repulsion. The same computation predicts a much faster reacting IBX derivative with a 100 fold reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
when this ortho hydrogen atom is replaced by a methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
group thus facilitating the twist until the elimination reaction takes prevalence as the rate determining step.
IBX exists as two tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the reloca ...
s, one of which is the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
. The acidity
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
of IBX which has been determined in water (pKa
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:H ...
2.4) and DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
(pKa 6.65) is known to affect organic reactions, for instance acid-catalyzed isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
accompanying oxidations.
Scope
IBX is also available assilica gel
Silica gel is an amorphous and porosity, porous form of silicon dioxide (silica), consisting of an irregular three-dimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain wate ...
or polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
bound IBX. In many applications, IBX is replaced by Dess–Martin periodinane
Dess–Martin periodinane (DMP) is a chemical reagent used in the Dess–Martin oxidation, Alcohol oxidation, oxidizing primary alcohols to aldehydes and secondary Alcohol (chemistry), alcohols to ketones. This periodinane has several advantages o ...
which is more soluble in common organic solvents. A sample reaction is an IBX oxidation used in the total synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of eicosanoid
Eicosanoids are lipid signaling, signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosa ...
: More and Finney and Van Arman have demonstrated that common organic solvents are suitable for many IBX oxidations, despite its low solubility, and in fact may simplify product purification.
:
In 2001, K. C. Nicolaou and co-workers published a series of papers in the ''Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'' demonstrating, among other transformations, the use of IBX to oxidize primary and secondary benzylic carbons to aromatic aldehydes and ketones, respectively. The presence of additional water in the solution is now considered to be an essential part of those oxidations.
Oxidative cleavage
IBX is notable for oxidizing vicinal diols (or glycols) to diketones without cleavage of the carbon-carbon bond, but oxidative cleavage of glycols to two aldehydes or ketones can occur when modified conditions are used (elevated temperatures or trifluoroacetic acid solvent). : The reaction mechanism for thisglycol cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a vicinal diol (glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substituti ...
is based on initial formation of an adduct between 10-I-4 IBX and DMSO to a 12-I-5 intermediate 3 in which DMSO acts as a leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
for incoming alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
4 to intermediate 5. One equivalent of water is split off forming 12-I-5 spirobicyclic periodinane 6 setting the stage for fragmentation to 7. With hydroxyl alpha protons present, oxidation to the acyloin
In organic chemistry, acyloins or α-hydroxy ketones are a class of organic compounds of the general form , composed of a hydroxy group () adjacent to a ketone group (). The name ''acyloin'' is derived from the fact that they are formally deri ...
competes. Trifluoroacetic acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not ...
is found to facilitate the overall reaction.
:
α-Hydroxylations
Kirsch and co-workers were able to hydroxylate keto compounds with IBX in α-position under mild conditions. This method could be extended to β-keto esters, and forms a regioselective way to introduce conjugatively activated unsaturation.Oxidation of β-hydroxyketones to β-diketones
Bartlett and Beaudry discovered that IBX is a valuable reagent for the transformation of β-hydroxyketones to β-diketones. IBX provides yields superior to both the Swern and Dess–Martin oxidation protocols.References
{{DEFAULTSORT:Iodoxybenzoic acid, 2- Iodates Periodinanes Iodine heterocycles Oxygen heterocycles Reagents for organic chemistry Oxidizing acids Heterocyclic compounds with 2 rings Lactones