hypervalence on:
[Wikipedia]
[Google]
[Amazon]
In
Link
/ref> Lewis maintained the importance of the two-center two-electron (2c–2e) bond in describing hypervalence, thus using expanded octets to account for such molecules. Using the language of orbital hybridization, the bonds of molecules like PF5 and SF6 were said to be constructed from sp3dn orbitals on the central atom. Langmuir, on the other hand, upheld the dominance of the octet rule and preferred the use of ionic bonds to account for hypervalence without violating the rule (e.g. " 2F−" for SF6). In the late 1920s and 1930s, Sugden argued for the existence of a two-center one-electron (2c–1e) bond and thus rationalized bonding in hypervalent molecules without the need for expanded octets or ionic bond character; this was poorly accepted at the time. In the 1940s and 1950s, Rundle and Pimentel popularized the idea of the three-center four-electron bond, which is essentially the same concept which Sugden attempted to advance decades earlier; the three-center four-electron bond can be alternatively viewed as consisting of two collinear two-center one-electron bonds, with the remaining two nonbonding electrons localized to the ligands. The attempt to actually prepare hypervalent organic molecules began with

 This led the authors to the interesting conclusion that "Contrary to what we were all taught as undergraduates, the nitrogen atom does indeed form five covalent linkages and the availability or otherwise of d-orbitals has nothing to do with this state of affairs."
This led the authors to the interesting conclusion that "Contrary to what we were all taught as undergraduates, the nitrogen atom does indeed form five covalent linkages and the availability or otherwise of d-orbitals has nothing to do with this state of affairs."

chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
that contains one or more main group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s apparently bearing more than eight electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in their valence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s. Phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
(), sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride ( British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attache ...
(), chlorine trifluoride (), the chlorite
The chlorite ion, or chlorine dioxide anion, is the halite (oxyanion), halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as s ...
() ion in chlorous acid
Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl ...
and the triiodide
In chemistry, triiodide usually refers to the triiodide ion, . This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been ...
() ion are examples of hypervalent molecules.
Definitions and nomenclature
Hypervalent molecules were first formally defined by Jeremy I. Musher in 1969 as molecules having central atoms of group 15–18 in any valence other than the lowest (i.e. 3, 2, 1, 0 for Groups 15, 16, 17, 18 respectively, based on theoctet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
).
Several specific classes of hypervalent molecules exist:
* Hypervalent iodine compounds are useful reagents in organic chemistry (e.g. Dess–Martin periodinane)
* Tetra-, penta- and hexavalent phosphorus, silicon, and sulfur compounds (e.g. PCl5, PF5, SF6, sulfuranes and persulfuranes)
* Noble gas compounds (ex. xenon tetrafluoride, XeF4)
* Halogen polyfluorides (ex. chlorine pentafluoride, ClF5)
N-X-L notation
N-X-L nomenclature, introduced collaboratively by the research groups ofMartin Martin may refer to:
Places Antarctica
* Martin Peninsula, Marie Byrd Land
* Port Martin, Adelie Land
* Point Martin, South Orkney Islands
Europe
* Martin, Croatia, a village
* Martin, Slovakia, a city
* Martín del Río, Aragón, Spain
* M ...
, Arduengo, and Kochi
Kochi ( , ), List of renamed Indian cities and states#Kerala, formerly known as Cochin ( ), is a major port city along the Malabar Coast of India bordering the Laccadive Sea. It is part of the Ernakulam district, district of Ernakulam in the ...
in 1980, is often used to classify hypervalent compounds of main group elements, where:
* N represents the number of valence electrons
* X is the chemical symbol of the central atom
* L the number of ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s to the central atom
Examples of N-X-L nomenclature include:
* XeF2, 10-Xe-2
* PCl5, 10-P-5
* SF6, 12-S-6
* IF7, 14-I-7
History and controversy
The debate over the nature and classification of hypervalent molecules goes back toGilbert N. Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a dean of the college of chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bon ...
and Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and metallurgical engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publicatio ...
and the debate over the nature of the chemical bond in the 1920s. Link
/ref> Lewis maintained the importance of the two-center two-electron (2c–2e) bond in describing hypervalence, thus using expanded octets to account for such molecules. Using the language of orbital hybridization, the bonds of molecules like PF5 and SF6 were said to be constructed from sp3dn orbitals on the central atom. Langmuir, on the other hand, upheld the dominance of the octet rule and preferred the use of ionic bonds to account for hypervalence without violating the rule (e.g. " 2F−" for SF6). In the late 1920s and 1930s, Sugden argued for the existence of a two-center one-electron (2c–1e) bond and thus rationalized bonding in hypervalent molecules without the need for expanded octets or ionic bond character; this was poorly accepted at the time. In the 1940s and 1950s, Rundle and Pimentel popularized the idea of the three-center four-electron bond, which is essentially the same concept which Sugden attempted to advance decades earlier; the three-center four-electron bond can be alternatively viewed as consisting of two collinear two-center one-electron bonds, with the remaining two nonbonding electrons localized to the ligands. The attempt to actually prepare hypervalent organic molecules began with
Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
and Georg Wittig in the first half of the twentieth century, who sought to challenge the extant valence theory and successfully prepare nitrogen and phosphorus-centered hypervalent molecules. The theoretical basis for hypervalency was not delineated until J.I. Musher's work in 1969.
In 1990, Magnusson published a seminal work definitively excluding the significance of d-orbital hybridization in the bonding of hypervalent compounds of second-row elements. This had long been a point of contention and confusion in describing these molecules using molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, whic ...
. Part of the confusion here originates from the fact that one must include d-functions in the basis sets used to describe these compounds (or else unreasonably high energies and distorted geometries result), and the contribution of the d-function to the molecular wavefunction is large. These facts were historically interpreted to mean that d-orbitals must be involved in bonding. However, Magnusson concludes in his work that d-orbital involvement is not implicated in hypervalency.
Nevertheless, a 2013 study showed that although the Pimentel ionic model best accounts for the bonding of hypervalent species, the energetic contribution of an expanded octet structure is also not null. In this modern valence bond theory study of the bonding of xenon difluoride
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as th ...
, it was found that ionic structures account for about 81% of the overall wavefunction, of which 70% arises from ionic structures employing only the p orbital on xenon while 11% arises from ionic structures employing an hybrid on xenon. The contribution of a formally hypervalent structure employing an orbital of sp3d hybridization on xenon accounts for 11% of the wavefunction, with a diradical contribution making up the remaining 8%. The 11% sp3d contribution results in a net stabilization of the molecule by mol−1, a minor but significant fraction of the total energy of the total bond energy ( mol−1). Other studies have similarly found minor but non-negligible energetic contributions from expanded octet structures in SF6 (17%) and XeF6 (14%).
Despite the lack of chemical realism, the IUPAC recommends the drawing of expanded octet structures for functional groups like sulfones and phosphoranes, in order to avoid the drawing of a large number of formal charges or partial single bonds.
Hypervalent hydrides
A special type of hypervalent molecules is hypervalent hydrides. Most known hypervalent molecules contain substituents more electronegative than their central atoms. Hypervalent hydrides are of special interest because hydrogen is usually less electronegative than the central atom. A number of computational studies have been performed on chalcogen hydrides andpnictogen hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, bismuth, and moscovium) covalently bonded ...
s. Recently, a new computational study has shown that most hypervalent halogen hydrides XHn can exist. It is suggested that IH3 and IH5 are stable enough to be observable or, possibly, even isolable.
Criticism
Both the term and concept of hypervalency still fall under criticism. In 1984, in response to this general controversy, Paul von Ragué Schleyer proposed the replacement of 'hypervalency' with use of the term hypercoordination because this term does not imply any mode of chemical bonding and the question could thus be avoided altogether. The concept itself has been criticized by Ronald Gillespie who, based on an analysis of electron localization functions, wrote in 2002 that "as there is no fundamental difference between the bonds in hypervalent and non-hypervalent (Lewis octet) molecules there is no reason to continue to use the term hypervalent." For hypercoordinated molecules withelectronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
ligands such as PF5, it has been demonstrated that the ligands can pull away enough electron density from the central atom so that its net content is again 8 electrons or fewer. Consistent with this alternative view is the finding that hypercoordinated molecules based on fluorine ligands, for example PF5 do not have hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
counterparts, e.g. phosphorane (PH5) which is unknown.
The ionic model holds up well in thermochemical calculations. It predicts favorable exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
formation of from phosphorus trifluoride PF3 and fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
F2 whereas a similar reaction forming is not favorable.
Alternative definition
Durrant has proposed an alternative definition of hypervalency, based on the analysis of atomic charge maps obtained from atoms in molecules theory. This approach defines a parameter called the valence electron equivalent, γ, as “the formal shared electron count at a given atom, obtained by any combination of valid ionic and covalent resonance forms that reproduces the observed charge distribution”. For any particular atom X, if the value of γ(X) is greater than 8, that atom is hypervalent. Using this alternative definition, many species such as PCl5, , and XeF4, that are hypervalent by Musher's definition, are reclassified as hypercoordinate but not hypervalent, due to strongly ionic bonding that draws electrons away from the central atom. On the other hand, some compounds that are normally written with ionic bonds in order to conform to the octet rule, such asozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
O3, nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
NNO, and trimethylamine N-oxide , are found to be genuinely hypervalent. Examples of γ calculations for phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
(γ(P) = 2.6, non-hypervalent) and orthonitrate (γ(N) = 8.5, hypervalent) are shown below.

Bonding in hypervalent molecules
Early considerations of the geometry of hypervalent molecules returned familiar arrangements that were well explained by the VSEPR model for atomic bonding. Accordingly, AB5 and AB6 type molecules would possess a trigonal bi-pyramidal and octahedral geometry, respectively. However, in order to account for the observed bond angles, bond lengths and apparent violation of the Lewisoctet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
, several alternative models have been proposed.
In the 1950s an expanded valence shell treatment of hypervalent bonding was adduced to explain the molecular architecture, where the central atom of penta- and hexacoordinated molecules would utilize d AOs in addition to s and p AOs. However, advances in the study of ''ab initio
( ) is a Latin term meaning "from the beginning" and is derived from the Latin ("from") + , ablative singular of ("beginning").
Etymology
, from Latin, literally "from the beginning", from ablative case of "entrance", "beginning", related t ...
'' calculations have revealed that the contribution of d-orbitals to hypervalent bonding is too small to describe the bonding properties, and this description is now regarded as much less important. It was shown that in the case of hexacoordinated SF6, d-orbitals are not involved in S-F bond formation, but charge transfer between the sulfur and fluorine atoms and the apposite resonance structures were able to account for the hypervalency (See below).
Additional modifications to the octet rule have been attempted to involve ionic characteristics in hypervalent bonding. As one of these modifications, in 1951, the concept of the 3-center 4-electron (3c-4e) bond, which described hypervalent bonding with a qualitative molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
, was proposed. The 3c-4e bond is described as three molecular orbitals given by the combination of a p atomic orbital on the central atom and an atomic orbital from each of the two ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s on opposite sides of the central atom. Only one of the two pairs of electrons is occupying a molecular orbital that involves bonding to the central atom, the second pair being non-bonding and occupying a molecular orbital composed of only atomic orbitals from the two ligands. This model in which the octet rule is preserved was also advocated by Musher.
image:XeF2.svg, 400px, center, Qualitative model for a three-center four-electron bond
Molecular orbital theory
A complete description of hypervalent molecules arises from consideration of molecular orbital theory through quantum mechanical methods. An LCAO in, for example, sulfur hexafluoride, taking a basis set of the one sulfur 3s-orbital, the three sulfur 3p-orbitals, and six octahedral geometry symmetry-adapted linear combinations (SALCs) of fluorine orbitals, a total of ten molecular orbitals are obtained (four fully occupied bonding MOs of the lowest energy, two fully occupied intermediate energy non-bonding MOs and four vacant antibonding MOs with the highest energy) providing room for all 12 valence electrons. This is a stable configuration only for S''X''6 molecules containing electronegative ligand atoms like fluorine, which explains why SH6 is not a stable molecule. In the bonding model, the two non-bonding MOs (1eg) are localized equally on all six fluorine atoms.Valence bond theory
For hypervalent compounds in which the ligands are moreelectronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than the central, hypervalent atom, resonance structures can be drawn with no more than four covalent electron pair bonds and completed with ionic bonds to obey the octet rule. For example, in phosphorus pentafluoride (PF5), 5 resonance structures can be generated each with four covalent bonds and one ionic bond with greater weight in the structures placing ionic character in the axial bonds, thus satisfying the octet rule and explaining both the observed trigonal bipyramidal molecular geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not ident ...
and the fact that the axial bond length (158 pm) is longer than the equatorial (154 pm).
500px , center , Phosphorus pentafluoride. There are 2 possible structures with an axial ionic bond, plus 3 possible structures with an equatorial ionic bond.
For a hexacoordinate molecule such as sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride ( British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attache ...
, each of the six bonds is the same length. The rationalization described above can be applied to generate 15 resonance structures each with four covalent bonds and two ionic bonds, such that the ionic character is distributed equally across each of the sulfur-fluorine bonds.
image:hexa sulf.svg, 500px , center , Sulfur hexafluoride. There are 12 structures with the two ionic bonds in adjacent (''cis'') positions, plus 3 structures with the two ionic bonds in opposite (''trans'') positions.
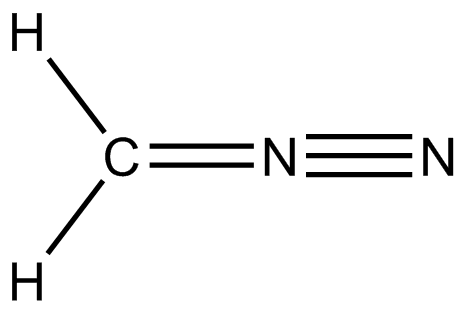
Spin-coupled valence bond theory has been applied to diazomethane and the resulting orbital analysis was interpreted in terms of a chemical structure in which the central nitrogen has five covalent bonds;
 This led the authors to the interesting conclusion that "Contrary to what we were all taught as undergraduates, the nitrogen atom does indeed form five covalent linkages and the availability or otherwise of d-orbitals has nothing to do with this state of affairs."
This led the authors to the interesting conclusion that "Contrary to what we were all taught as undergraduates, the nitrogen atom does indeed form five covalent linkages and the availability or otherwise of d-orbitals has nothing to do with this state of affairs."
Structure, reactivity, and kinetics
Structure
Hexacoordinated phosphorus
Hexacoordinatephosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
molecules involving nitrogen, oxygen, or sulfur ligands provide examples of Lewis acid-Lewis base hexacoordination. For the two similar complexes shown below, the length of the C–P bond increases with decreasing length of the N–P bond; the strength of the C–P bond decreases with increasing strength of the N–P Lewis acid–Lewis base interaction.
image:hexa phos.png, 300px , center , Relative bond strengths in hexacoordinated phosphorus compounds. In A, the N–P bond is 1.980 Å long and the C–P is 1.833 Å long, and in B, the N–P bond increases to 2.013 Å as the C–P bond decreases to 1.814 Å.
Pentacoordinated silicon
This trend is also generally true of pentacoordinated main-group elements with one or more lone-pair-containing ligand, including the oxygen-pentacoordinatedsilicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
examples shown below.
image:penta sil oxy.png, 500px , center , Relative bond strengths in pentacoordinated silicon compounds. In A, the Si-O bond length is 1.749Å and the Si-I bond length is 3.734Å; in B, the Si-O bond lengthens to 1.800Å and the Si-Br bond shortens to 3.122Å, and in C, the Si-O bond is the longest at 1.954Å and the Si-Cl bond the shortest at 2.307A.
The Si-halogen bonds range from close to the expected van der Waals value in A (a weak bond) almost to the expected covalent single bond value in C (a strong bond).
Reactivity
Silicon
Corriu and coworkers performed early work characterizing reactions thought to proceed through a hypervalent transition state. Measurements of thereaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
s of hydrolysis of tetravalent chlorosilanes incubated with catalytic amounts of water returned a rate that is first order
In mathematics and other formal sciences, first-order or first order most often means either:
* "linear" (a polynomial of degree at most one), as in first-order approximation and other calculus uses, where it is contrasted with "polynomials of high ...
in chlorosilane and second order in water. This indicated that two water molecules interacted with the silane during hydrolysis and from this a binucleophilic reaction mechanism was proposed. Corriu and coworkers then measured the rates of hydrolysis in the presence of nucleophilic catalyst HMPT, DMSO or DMF. It was shown that the rate of hydrolysis was again first order in chlorosilane, first order in catalyst and now first order in water. Appropriately, the rates of hydrolysis also exhibited a dependence on the magnitude of charge on the oxygen of the nucleophile.
Taken together this led the group to propose a reaction mechanism in which there is a pre-rate determining nucleophilic attack of the tetracoordinated silane by the nucleophile (or water) in which a hypervalent pentacoordinated silane is formed. This is followed by a nucleophilic attack of the intermediate by water in a rate determining step leading to hexacoordinated species that quickly decomposes giving the hydroxysilane.
Silane hydrolysis was further investigated by Holmes and coworkers in which tetracoordinated (Mes = mesityl) and pentacoordinated were reacted with two equivalents of water. Following twenty-four hours, almost no hydrolysis of the tetracoordinated silane was observed, while the pentacoordinated silane was completely hydrolyzed after fifteen minutes. Additionally, X-ray diffraction data collected for the tetraethylammonium salts of the fluorosilanes showed the formation of hydrogen bisilonate lattice supporting a hexacoordinated intermediate from which is quickly displaced leading to the hydroxylated product. This reaction and crystallographic data support the mechanism proposed by Corriu ''et al.''.
500px , center , Mechanism of silane hydrolysis and structure of the hydrogen bisilonate lattice
The apparent increased reactivity of hypervalent molecules, contrasted with tetravalent analogues, has also been observed for Grignard reactions. The Corriu group measured Grignard reaction half-times by NMR for related 18-crown-6 potassium salts of a variety of tetra- and pentacoordinated fluorosilanes in the presence of catalytic amounts of nucleophile.
Though the half reaction method is imprecise, the magnitudinal differences in reactions rates allowed for a proposed reaction scheme wherein, a pre-rate determining attack of the tetravalent silane by the nucleophile results in an equilibrium between the neutral tetracoordinated species and the anionic pentavalent compound. This is followed by nucleophilic coordination by two Grignard reagents as normally seen, forming a hexacoordinated transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
and yielding the expected product.
500px , center , Grignard reaction mechanism for tetracoordinate silanes and the analogous hypervalent pentacoordinated silanes
The mechanistic implications of this are extended to a hexacoordinated silicon species that is thought to be active as a transition state in some reactions. The reaction of allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
- or crotyl-trifluorosilanes with aldehydes and ketones only precedes with fluoride activation to give a pentacoordinated silicon. This intermediate then acts as a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
to coordinate with the carbonyl oxygen atom. The further weakening of the silicon–carbon bond as the silicon becomes hexacoordinate helps drive this reaction.
Phosphorus
Similar reactivity has also been observed for other hypervalent structures such as the miscellany of phosphorus compounds, for which hexacoordinated transition states have been proposed. Hydrolysis of phosphoranes and oxyphosphoranes have been studied and shown to be second order in water. Bel'skii ''et al.''. have proposed a prerate determining nucleophilic attack by water resulting in an equilibrium between the penta- and hexacoordinated phosphorus species, which is followed by a proton transfer involving the second water molecule in a rate determining ring-opening step, leading to the hydroxylated product. 500px , center , Mechanism of the hydrolysis of pentacoordinated phosphorus Alcoholysis of pentacoordinated phosphorus compounds, such as trimethoxyphospholene with benzyl alcohol, have also been postulated to occur through a similar octahedral transition state, as in hydrolysis, however without ring opening. 500px , center , Mechanism of the base catalyzed alcoholysis of pentacoordinated phosphorus It can be understood from these experiments that the increased reactivity observed for hypervalent molecules, contrasted with analogous nonhypervalent compounds, can be attributed to the congruence of these species to the hypercoordinated activated states normally formed during the course of the reaction.Ab initio calculations
The enhanced reactivity at pentacoordinated silicon is not fully understood. Corriu and coworkers suggested that greater electropositive character at the pentavalent silicon atom may be responsible for its increased reactivity. Preliminary ab initio calculations supported this hypothesis to some degree, but used a small basis set. A software program for ab initio calculations, Gaussian 86, was used by Dieters and coworkers to compare tetracoordinated silicon and phosphorus to their pentacoordinate analogues. Thisab initio
( ) is a Latin term meaning "from the beginning" and is derived from the Latin ("from") + , ablative singular of ("beginning").
Etymology
, from Latin, literally "from the beginning", from ablative case of "entrance", "beginning", related t ...
approach is used as a supplement to determine why reactivity improves in nucleophilic reactions with pentacoordinated compounds. For silicon, the 6-31+G* basis set was used because of its pentacoordinated anionic character and for phosphorus, the 6-31G* basis set was used.
Pentacoordinated compounds should theoretically be less electrophilic than tetracoordinated analogues due to steric hindrance and greater electron density from the ligands, yet experimentally show greater reactivity with nucleophiles than their tetracoordinated analogues. Advanced ab initio calculations were performed on series of tetracoordinated and pentacoordinated species to further understand this reactivity phenomenon. Each series varied by degree of fluorination. Bond lengths and charge densities are shown as functions of how many hydride ligands are on the central atoms. For every new hydride, there is one less fluoride.
For silicon and phosphorus bond lengths, charge densities, and Mulliken bond overlap, populations were calculated for tetra and pentacoordinated species by this ab initio approach. Addition of a fluoride ion to tetracoordinated silicon shows an overall average increase of 0.1 electron charge, which is considered insignificant. In general, bond lengths in trigonal bipyramidal pentacoordinate species are longer than those in tetracoordinate analogues. Si-F bonds and Si-H bonds both increase in length upon pentacoordination and related effects are seen in phosphorus species, but to a lesser degree. The reason for the greater magnitude in bond length change for silicon species over phosphorus species is the increased effective nuclear charge at phosphorus. Therefore, silicon is concluded to be more loosely bound to its ligands.
In addition Dieters and coworkers show an inverse correlation between bond length and bond overlap for all series. Pentacoordinated species are concluded to be more reactive because of their looser bonds as trigonal-bipyramidal structures.
By calculating the energies for the addition and removal of a fluoride ion in various silicon and phosphorus species, several trends were found. In particular, the tetracoordinated species have much higher energy requirements for ligand removal than do pentacoordinated species. Further, silicon species have lower energy requirements for ligand removal than do phosphorus species, which is an indication of weaker bonds in silicon.
See also
*Charge-shift bond
In theoretical chemistry, the charge-shift bond is a proposed new class of chemical bonds that sits alongside the three familiar families of covalent bond, covalent, ionic bond, ionic, and metallic bonds where electrons are shared or transferred r ...
References
External links
* {{DEFAULTSORT:Hypervalent Molecule Chemical bonding Molecular geometry