hydroboration–oxidation reaction on:
[Wikipedia]
[Google]
[Amazon]
Hydroboration–oxidation reaction is a two-step 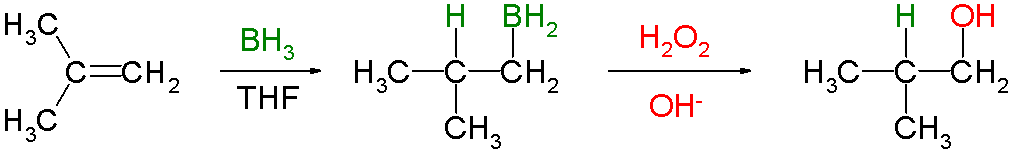
 Knowing that the group containing the boron will be replaced by a hydroxyl group, it can be seen that the initial hydroboration step determines the regioselectivity. Hydroboration proceeds in an anti-Markovnikov manner. The reaction sequence is also stereospecific, giving syn addition (on the same face of the alkene): the hydroboration is syn-selective and the oxidation replaces the boron with hydroxyl having the same geometric position. Thus 1-methylcyclopentene reacts with diborane predominantly to give ''trans''-1-hydroxy-2-methylcyclopentane—the newly added H and OH are ''cis'' to each other.
Until all hydrogens attached to boron have been transferred away, the boron group BH2 will continue adding to more alkenes. This means that one mole of hydroborane will undergo the reaction with three moles of alkene. Furthermore, it is not necessary for the hydroborane to have more than one hydrogen. For example, reagents of the type R2BH are commonly used, where R can represents the remainder of the molecule. Such modified hydroboration reagents include 9-BBN, catecholborane, and disiamylborane.
Knowing that the group containing the boron will be replaced by a hydroxyl group, it can be seen that the initial hydroboration step determines the regioselectivity. Hydroboration proceeds in an anti-Markovnikov manner. The reaction sequence is also stereospecific, giving syn addition (on the same face of the alkene): the hydroboration is syn-selective and the oxidation replaces the boron with hydroxyl having the same geometric position. Thus 1-methylcyclopentene reacts with diborane predominantly to give ''trans''-1-hydroxy-2-methylcyclopentane—the newly added H and OH are ''cis'' to each other.
Until all hydrogens attached to boron have been transferred away, the boron group BH2 will continue adding to more alkenes. This means that one mole of hydroborane will undergo the reaction with three moles of alkene. Furthermore, it is not necessary for the hydroborane to have more than one hydrogen. For example, reagents of the type R2BH are commonly used, where R can represents the remainder of the molecule. Such modified hydroboration reagents include 9-BBN, catecholborane, and disiamylborane.
 The 'H' atom in the reaction comes from B2H6, the 'O' atom comes from hydrogen peroxide (H2O2) whereas the O attached 'H' atom comes from the solvent (refer mechanism).
The 'H' atom in the reaction comes from B2H6, the 'O' atom comes from hydrogen peroxide (H2O2) whereas the O attached 'H' atom comes from the solvent (refer mechanism).
https://www.organic-chemistry.org/namedreactions/brown-hydroboration.shtm
{{DEFAULTSORT:Hydroboration-oxidation reaction Addition reactions
hydration reaction
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed indust ...
that converts an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
into an alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
. The process results in the syn addition of a hydrogen and a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group where the double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
in 1979.
The general form of the reaction is as follows:

Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) is the archetypal solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
used for hydroboration.
Mechanism and scope
Hydroboration step
In the first step,borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a c ...
(BH3) adds to the double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
, transferring one of the hydrogen atoms to the carbon adjacent to the one that becomes bonded to the boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
. This hydroboration is repeated two additional times, successively reacting each B–H bond so that three alkenes add to each BH3. The resulting trialkylborane is treated with hydrogen peroxide in the second step. This process replaces the B-C bonds with HO-C bonds. The boron reagent is converted to boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white ...
. The reaction was originally described by H.C. Brown in 1957 for the conversion of 1-hexene into 1-hexanol.
Oxidation step
In the second step of the reaction sequence, the nucleophilichydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anio ...
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
attacks the boron atom. Alkyl migration to oxygen gives the alkyl borane with retention of stereochemistry (in reality, the reaction occurs via the trialkyl borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
B(OR)3, rather than the monoalkyl borinic ester BH2OR).
Alkyne hydroboration
A hydroboration reaction also takes place onalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s. Again the mode of action is ''syn'' and secondary reaction products are aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s from terminal alkynes and ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s from internal alkynes. In order to prevent hydroboration across both the pi-bonds, a bulky borane like disiamyl (di-sec-iso-amyl) borane is used.
Alternative oxidations
Use of other oxidants instead of hydrogen peroxide can lead to carbonyl products rather than alcohols from alkenes. ''N''-Methylmorpholine ''N''-oxide with catalytic tetrapropylammonium perruthenate converts the alkylborane into a carbonyl, thus a ketone oraldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
product depending on what other groups were attached to that carbon in the original alkene. Various dichromates or related chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
(VI) reagents give ketones as well, but give carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s instead of aldehydes for terminal alkenes.
Other oxidation substrates
Aside from boranes, the oxidation of silanes and disilanes can also yield hydroxy groups. A major difference is that while silyl groups like the phenyldimethylsilyl group are converted to the hydroxy group after acid or otherelectrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
treatment followed by oxidation by hydrogen peroxide, disilanyl groups are converted after TBAF treatment followed by peroxide oxidation. This allows for selective oxidation of either group.
References
External links
* Organic Chemistry Portal. Hydroboration (including recent literature)https://www.organic-chemistry.org/namedreactions/brown-hydroboration.shtm
{{DEFAULTSORT:Hydroboration-oxidation reaction Addition reactions