Expanded polystyrene on:
[Wikipedia]
[Google]
[Amazon]

 Polystyrene (PS) is a synthetic
Polystyrene (PS) is a synthetic
 In
In
 Each carbon of the backbone has tetrahedral geometry, and those carbons that have a
Each carbon of the backbone has tetrahedral geometry, and those carbons that have a


 Polystyrene (PS) is used for producing disposable plastic cutlery and dinnerware, CD "jewel" cases, smoke detector housings, license plate frames,
Polystyrene (PS) is used for producing disposable plastic cutlery and dinnerware, CD "jewel" cases, smoke detector housings, license plate frames,
 Polystyrene foams are 95–98% air. Polystyrene foams are good thermal insulators and are therefore often used as building insulation materials, such as in insulating concrete forms and structural insulated panel building systems. Grey polystyrene foam, incorporating
Polystyrene foams are 95–98% air. Polystyrene foams are good thermal insulators and are therefore often used as building insulation materials, such as in insulating concrete forms and structural insulated panel building systems. Grey polystyrene foam, incorporating
 Extruded polystyrene foam (XPS) consists of closed cells. It offers improved surface roughness, higher stiffness and reduced thermal conductivity. The density range is about 28–34 kg/m3.
Extruded polystyrene material is also used in
Extruded polystyrene foam (XPS) consists of closed cells. It offers improved surface roughness, higher stiffness and reduced thermal conductivity. The density range is about 28–34 kg/m3.
Extruded polystyrene material is also used in
 Animals do not recognize polystyrene foam as an artificial material and may even mistake it for food.
Polystyrene foam blows in the wind and floats on water due to its low specific gravity. It can have serious effects on the health of birds and marine animals that swallow significant quantities. Juvenile rainbow trout exposed to polystyrene fragments show toxic effects in the form of substantial histomorphometrical changes.
Animals do not recognize polystyrene foam as an artificial material and may even mistake it for food.
Polystyrene foam blows in the wind and floats on water due to its low specific gravity. It can have serious effects on the health of birds and marine animals that swallow significant quantities. Juvenile rainbow trout exposed to polystyrene fragments show toxic effects in the form of substantial histomorphometrical changes.
 In general, polystyrene is not accepted in curbside collection recycling programs and is not separated and recycled where it is accepted. In Germany, polystyrene is collected as a consequence of the packaging law (Verpackungsverordnung) that requires manufacturers to take responsibility for recycling or disposing of any packaging material they sell.
Most polystyrene products are currently not recycled due to the lack of incentive to invest in the compactors and logistical systems required. Due to the low density of polystyrene foam, it is not economical to collect. However, if the waste material goes through an initial compaction process, the material changes density from typically 30 kg/m3 to 330 kg/m3 and becomes a recyclable commodity of high value for producers of recycled plastic pellets. Expanded polystyrene scrap can be easily added to products such as EPS insulation sheets and other EPS materials for construction applications; many manufacturers cannot obtain sufficient scrap because of collection issues. When it is not used to make more EPS, foam scrap can be turned into products such as clothes hangers, park benches, flower pots, toys, rulers, stapler bodies, seedling containers, picture frames, and architectural molding from recycled PS. As of 2016, around 100 tonnes of EPS are recycled every month in the UK.
Recycled EPS is also used in many metal casting operations. Rastra is made from EPS that is combined with cement to be used as an insulating amendment in the making of concrete foundations and walls. American manufacturers have produced insulating concrete forms made with approximately 80% recycled EPS since 1993.
In general, polystyrene is not accepted in curbside collection recycling programs and is not separated and recycled where it is accepted. In Germany, polystyrene is collected as a consequence of the packaging law (Verpackungsverordnung) that requires manufacturers to take responsibility for recycling or disposing of any packaging material they sell.
Most polystyrene products are currently not recycled due to the lack of incentive to invest in the compactors and logistical systems required. Due to the low density of polystyrene foam, it is not economical to collect. However, if the waste material goes through an initial compaction process, the material changes density from typically 30 kg/m3 to 330 kg/m3 and becomes a recyclable commodity of high value for producers of recycled plastic pellets. Expanded polystyrene scrap can be easily added to products such as EPS insulation sheets and other EPS materials for construction applications; many manufacturers cannot obtain sufficient scrap because of collection issues. When it is not used to make more EPS, foam scrap can be turned into products such as clothes hangers, park benches, flower pots, toys, rulers, stapler bodies, seedling containers, picture frames, and architectural molding from recycled PS. As of 2016, around 100 tonnes of EPS are recycled every month in the UK.
Recycled EPS is also used in many metal casting operations. Rastra is made from EPS that is combined with cement to be used as an insulating amendment in the making of concrete foundations and walls. American manufacturers have produced insulating concrete forms made with approximately 80% recycled EPS since 1993.
. Newton.dep.anl.gov. Retrieved 25 December 2011. Q and A page with an partially incorrect information. According to the American Chemistry Council, when polystyrene is incinerated in modern facilities, the final volume is 1% of the starting volume; most of the polystyrene is converted into carbon dioxide, water vapor, and heat. Because of the amount of heat released, it is sometimes used as a power source for steam or electricity generation. When polystyrene was burned at temperatures of 800–900 °C (the typical range of a modern incinerator), the products of combustion consisted of "a complex mixture of polycyclic aromatic hydrocarbons (PAHs) from alkyl benzenes to benzoperylene. Over 90 different compounds were identified in combustion effluents from polystyrene." The American National Bureau of Standards Center for Fire Research found 57 chemical by-products released during the combustion of expanded polystyrene (EPS) foam.
Polystyrene Composition
– The University of Southern Mississippi
SPI resin identification code
– Society of the Plastics Industry
Polystyrene: Local Ordinances
– Californians Against Waste
Take a Closer Look at Today's Polystyrene Packaging
(brochure by the industry group American Chemistry Council, arguing that the material is "safe, affordable and environmentally responsible") * {{Authority control Insulators Building insulation materials Organic polymers Packaging materials Food packaging Thermoplastics Commodity chemicals Vinyl polymers

 Polystyrene (PS) is a synthetic
Polystyrene (PS) is a synthetic polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
made from monomers of the aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
hydrocarbon styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
. Polystyrene can be solid or foam
Foams are two-phase materials science, material systems where a gas is dispersed in a second, non-gaseous material, specifically, in which gas cells are enclosed by a distinct liquid or solid material. Note, this source focuses only on liquid ...
ed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to air and water vapor and has a relatively low melting point. Polystyrene is one of the most widely used plastic
Plastics are a wide range of synthetic polymers, synthetic or Semisynthesis, semisynthetic materials composed primarily of Polymer, polymers. Their defining characteristic, Plasticity (physics), plasticity, allows them to be Injection moulding ...
s, with the scale of its production being several million tonnes per year. Polystyrene is naturally transparent to visible light, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and optical disc
An optical disc is a flat, usuallyNon-circular optical discs exist for fashion purposes; see shaped compact disc. disc-shaped object that stores information in the form of physical variations on its surface that can be read with the aid o ...
jewel cases), containers, lids, bottles, trays, tumblers, disposable
A disposable (also called disposable product) is a product designed for a single use after which it is recycled or is disposed as solid waste. The term is also sometimes used for products that may last several months (e.g. disposable air filt ...
cutlery, in the making of models, and as an alternative material for phonograph record
A phonograph record (also known as a gramophone record, especially in British English) or a vinyl record (for later varieties only) is an analog sound storage medium in the form of a flat disc with an inscribed, modulated spiral groove. The g ...
s.
As a thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
. It becomes rigid again when cooled. This temperature behaviour is exploited for extrusion
Extrusion is a process used to create objects of a fixed cross section (geometry), cross-sectional profile by pushing material through a Die (manufacturing), die of the desired cross-section. Its two main advantages over other manufacturing pro ...
(as in Styrofoam) and also for molding and vacuum forming
Vacuum forming is a simplified version of thermoforming, where a sheet of plastic in various forms of High Impact Polystyrene Sheet (HIPS) for low impact products, or ABS for bathroom shower trays, and HDPE for exterior vehicle parts, plus vari ...
, since it can be cast into molds with fine detail. The temperatures behavior can be controlled by photocrosslinking.
Under ASTM
ASTM International, formerly known as American Society for Testing and Materials, is a standards organization that develops and publishes voluntary consensus technical international standards for a wide range of materials, products, systems and s ...
standards, polystyrene is regarded as not biodegradable. It is accumulating as a form of litter
Litter consists of waste products that have been discarded incorrectly, without consent, at an unsuitable location. The waste is objects, often man-made, such as aluminum cans, paper cups, food wrappers, cardboard boxes or plastic bottles, but ...
in the outside environment, particularly along shores and waterways, especially in its foam form, and in the Pacific Ocean.
History
Polystyrene was discovered in 1839 by Eduard Simon, anapothecary
''Apothecary'' () is an Early Modern English, archaic English term for a medicine, medical professional who formulates and dispenses ''materia medica'' (medicine) to physicians, surgeons and patients. The modern terms ''pharmacist'' and, in Brit ...
from Berlin. From storax
Storax (; , ''stúrax''), often commercially sold as styrax, is a natural fragrant resin isolated from the wounded bark of ''Liquidambar orientalis'' Mill. (Asia Minor) and ''Liquidambar styraciflua'' L. (Eastern US, Mexico, Central America) (A ...
, the resin of the Oriental sweetgum tree '' Liquidambar orientalis'', he distilled an oily substance, that he named styrol, now called styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
. Several days later, Simon found that it had thickened into a jelly, now known to have been a polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
, that he dubbed styrol oxide ("Styroloxyd") because he presumed that it had resulted from oxidation ( styrene oxide is a distinct compound). By 1845 Jamaican-born chemist John Buddle Blyth and German chemist August Wilhelm von Hofmann showed that the same transformation of styrol took place in the absence of oxygen. They called the product "meta styrol"; analysis showed that it was chemically identical to Simon's Styroloxyd. In 1866 Marcellin Berthelot correctly identified the formation of meta styrol/Styroloxyd from styrol as a polymerisation process. About 80 years later it was realized that heating of styrol starts a chain reaction that produces macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physi ...
s, following the thesis of German organic chemist Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
(1881–1965). This eventually led to the substance receiving its present name, polystyrene.
The company I. G. Farben began manufacturing polystyrene in Ludwigshafen
Ludwigshafen, officially Ludwigshafen am Rhein (; meaning "Ludwig I of Bavaria, Ludwig's Port upon the Rhine"; Palatine German dialects, Palatine German: ''Ludwichshafe''), is a List of cities and towns in Germany, city in the German state of Rh ...
, about 1931, hoping it would be a suitable replacement for die-cast zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
in many applications. Success was achieved when they developed a reactor vessel that extruded polystyrene through a heated tube and cutter, producing polystyrene in pellet form.
Ray McIntire (1918–1996), a chemical engineer of Dow Chemical, rediscovered a process first patented in early 1930s by Swedish inventor Carl Munters. According to the Science History Institute, "Dow bought the rights to Munters's method and began producing a lightweight, water-resistant, and buoyant material that seemed perfectly suited for building docks and watercraft and for insulating homes, offices, and chicken sheds." In 1944, Styrofoam was patented.
Before 1949, chemical engineer Fritz Stastny (1908–1985) developed pre-expanded PS beads by incorporating aliphatic hydrocarbons, such as pentane. These beads are the raw material for molding parts or extruding sheets. BASF
BASF SE (), an initialism of its original name , is a European Multinational corporation, multinational company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters are located in Ludwigshafen, Ge ...
and Stastny applied for a patent that was issued in 1949. The molding process was demonstrated at the Kunststoff Messe 1952 in Düsseldorf. Products were named Styropor.
The crystal structure of isotactic polystyrene was reported by Giulio Natta.
In 1954, the Koppers Company in Pittsburgh
Pittsburgh ( ) is a city in Allegheny County, Pennsylvania, United States, and its county seat. It is the List of municipalities in Pennsylvania#Municipalities, second-most populous city in Pennsylvania (after Philadelphia) and the List of Un ...
, Pennsylvania, developed expanded polystyrene (EPS) foam under the trade name Dylite. In 1960, Dart Container, the largest manufacturer of foam cups, shipped their first order.
Structure and production
chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
terms, polystyrene is a long chain hydrocarbon wherein alternating carbon centers are attached to phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
s (a derivative of benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
). Polystyrene's chemical formula is ; it contains the chemical elements
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in i ...
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
.
The material's properties are determined by short-range van der Waals attractions between polymer chains. Since the molecules consist of thousands of atoms, the cumulative attractive force between the molecules is large. When heated (or deformed at a rapid rate, due to a combination of viscoelastic and thermal insulation properties), the chains can take on a higher degree of confirmation and slide past each other. This intermolecular weakness (versus the high '' intramolecular'' strength due to the hydrocarbon backbone) confers flexibility and elasticity. The ability of the system to be readily deformed above its glass transition temperature allows polystyrene (and thermoplastic polymers in general) to be readily softened and molded upon heating. Extruded polystyrene is about as strong as an unalloyed aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
but much more flexible and much less dense (1.05 g/cm3 for polystyrene vs. 2.70 g/cm3 for aluminium).
Production
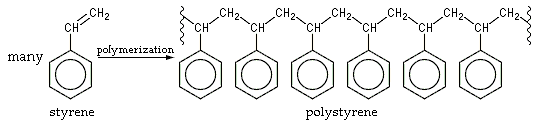
Polystyrene is an addition polymer that results when styrenemonomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
(interconnect). In the polymerization, the carbon-carbon π bond of the vinyl group is broken and a new carbon-carbon σ bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diatomi ...
is formed, attaching to the carbon of another styrene monomer to the chain. Since only one kind of monomer is used in its preparation, it is a homopolymer. The newly formed σ bond is stronger than the π bond that was broken, thus it is difficult to depolymerize polystyrene. About a few thousand monomers typically comprise a chain of polystyrene, giving a molar mass
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance ...
of 100,000–400,000 g/mol.
phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
(benzene ring) attached are stereogenic
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
. If the backbone were to be laid as a flat elongated zig-zag chain, each phenyl group would be tilted forward or backward compared to the plane of the chain.
The relative stereochemical relationship of consecutive phenyl groups determines the tacticity, which affects various physical properties of the material.
Tacticity
In polystyrene, tacticity describes the extent to which the phenyl group is uniformly aligned (arranged at one side) in the polymer chain. Tacticity has a strong effect on the properties of the plastic. Standard polystyrene is atactic. Thediastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
where all of the phenyl groups are on the same side is called ''isotactic'' polystyrene, which is not produced commercially.
Atactic polystyrene
The only commercially important form of polystyrene is ''atactic'', in which the phenyl groups are randomly distributed on both sides of the polymer chain. This random positioning prevents the chains from aligning with sufficient regularity to achieve any crystallinity. The plastic has a glass transition temperature ''T''g of ≈90 °C. Polymerization is initiated withfree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
s.Maul, J.; Frushour, B. G.; Kontoff, J. R.; Eichenauer, H.; Ott, K.-H. and Schade, C. (2007) "Polystyrene and Styrene Copolymers" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim,
Syndiotactic polystyrene
Ziegler–Natta polymerization can produce an ordered ''syndiotactic'' polystyrene with the phenyl groups positioned on alternating sides of the hydrocarbon backbone. This form is highly crystalline with a ''T''m (melting point) of . Syndiotactic polystyrene resin is currently produced under the trade name XAREC by Idemitsu corporation, who use a metallocene catalyst for the polymerisation reaction.Degradation
Polystyrene is relatively chemically inert. While it is waterproof and resistant to breakdown by many acids and bases, it is easily attacked by many organic solvents (e.g. it dissolves quickly when exposed toacetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
), chlorinated solvents, and aromatic hydrocarbon solvents. Because of its resilience and inertness, it is used for fabricating many objects of commerce. Like other organic compounds, polystyrene burns to give carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
, in addition to other thermal degradation by-products. Polystyrene, being an aromatic hydrocarbon
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
, typically combusts incompletely as indicated by the soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. Soot is considered a hazardous substance with carcinogenic properties. Most broadly, the term includes all the particulate matter produced b ...
y flame.
The process of depolymerizing polystyrene into its monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
, styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
, is called pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
. This involves using high heat and pressure to break down the chemical bonds between each styrene compound. Pyrolysis usually goes up to 430 °C. The high energy cost of doing this has made commercial recycling of polystyrene back into styrene monomer difficult.
Organisms
Polystyrene is generally considered to be non-biodegradable. However, certain organisms are able to degrade it, albeit very slowly. In 2015, researchers discovered that mealworms, the larvae form of the darkling beetle ''Tenebrio molitor'', could digest and subsist healthily on a diet of EPS. About 100 mealworms could consume between 34 and 39 milligrams of this white foam in a day. The droppings of mealworm were found to be safe for use as soil for crops. In 2016, it was also reported that superworms ('' Zophobas morio'') may eat expanded polystyrene (EPS). A group of high school students in Ateneo de Manila University found that compared to ''Tenebrio molitor'' larvae, ''Zophobas morio'' larvae may consume greater amounts of EPS over longer periods of time. In 2022 scientists identified several bacterial genera, including ''Pseudomonas
''Pseudomonas'' is a genus of Gram-negative bacteria belonging to the family Pseudomonadaceae in the class Gammaproteobacteria. The 348 members of the genus demonstrate a great deal of metabolic diversity and consequently are able to colonize a ...
'', ''Rhodococcus
''Rhodococcus'' is a genus of aerobic, nonsporulating, nonmotile Gram-positive bacteria closely related to ''Mycobacterium'' and ''Corynebacterium''. While a few species are pathogenic, most are benign, and have been found to thrive in a broad ...
'' and ''Corynebacterium
''Corynebacterium'' () is a genus of Gram-positive bacteria and most are aerobic. They are bacilli (rod-shaped), and in some phases of life they are, more specifically, club-shaped, which inspired the genus name ('' coryneform'' means "club-s ...
'', in the gut of superworms that contain encoded enzymes associated with the degradation of polystyrene and the breakdown product styrene.
The bacterium ''Pseudomonas putida
''Pseudomonas putida'' is a Gram-negative, rod-shaped, saprophytic soil bacterium. It has a versatile metabolism and is amenable to genetic manipulation, making it a common organism used in research, bioremediation, and synthesis of chemicals and ...
'' is capable of converting styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
oil into the biodegradable plastic PHA. This may someday be of use in the effective disposing of polystyrene foam. It is worthy to note the polystyrene must undergo pyrolysis to turn into styrene oil.
Forms produced
Polystyrene is commonly injection molded, vacuum formed, or extruded, while expanded polystyrene is either extruded or molded in a special process. Polystyrenecopolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are som ...
are also produced; these contain one or more other monomers in addition to styrene. In recent years the expanded polystyrene composites with cellulose and starch have also been produced. Polystyrene is used in some polymer-bonded explosives (PBX).
Sheet or molded polystyrene

plastic model
image:South-Goodwin.jpg, 300px, A young boy starts painting an assembled plastic model of the South Goodwin Lightship
A plastic model kit, (wikt:plamodel, plamo in Eastern world, Eastern influenced parlance), is a consumer-grade plastic scale mo ...
assembly kits, and many other objects where a rigid, economical plastic is desired. Production methods include thermoforming
Thermoforming is a manufacturing process where a plastic sheet is heated to a pliable forming temperature, formed to a specific shape in a mold, and trimmed to create a usable product. The sheet, or "film" when referring to thinner gauges and cert ...
(vacuum forming
Vacuum forming is a simplified version of thermoforming, where a sheet of plastic in various forms of High Impact Polystyrene Sheet (HIPS) for low impact products, or ABS for bathroom shower trays, and HDPE for exterior vehicle parts, plus vari ...
) and injection molding
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
.
Polystyrene Petri dish
A Petri dish (alternatively known as a Petri plate or cell-culture dish) is a shallow transparent lidded dish that biologists use to hold growth medium in which cells can be cultured,R. C. Dubey (2014): ''A Textbook Of Biotechnology For Class- ...
es and other laboratory
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratories are found in a variety of settings such as schools ...
containers such as test tubes and microplates play an important role in biomedical research and science. For these uses, articles are almost always made by injection molding, and often sterilized post-molding, either by irradiation or by treatment with ethylene oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless ...
. Post-mold surface modification, usually with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
-rich plasmas, is often done to introduce polar groups. Much of modern biomedical research relies on the use of such products; they, therefore, play a critical role in pharmaceutical research.
Thin sheets of polystyrene are used in polystyrene film capacitors as it forms a very stable dielectric
In electromagnetism, a dielectric (or dielectric medium) is an Insulator (electricity), electrical insulator that can be Polarisability, polarised by an applied electric field. When a dielectric material is placed in an electric field, electric ...
, but has largely fallen out of use in favor of polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
.
Foams
graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, has superior insulation properties.
Carl Munters and John Gudbrand Tandberg of Sweden received a US patent for polystyrene foam as an insulation product in 1935 (USA patent number 2,023,204).
PS foams also exhibit good damping properties, therefore it is used widely in packaging. The trademark
A trademark (also written trade mark or trade-mark) is a form of intellectual property that consists of a word, phrase, symbol, design, or a combination that identifies a Good (economics and accounting), product or Service (economics), service f ...
Styrofoam by Dow Chemical Company is informally used (mainly US & Canada) for all foamed polystyrene products, although strictly it should only be used for "extruded closed-cell" polystyrene foams made by Dow Chemicals.
Foams are also used for non-weight-bearing architectural structures (such as ornamental pillars).
Expanded polystyrene (EPS)
Expanded polystyrene (EPS), commonly called "styrofoam", is a rigid and tough, closed-cellfoam
Foams are two-phase materials science, material systems where a gas is dispersed in a second, non-gaseous material, specifically, in which gas cells are enclosed by a distinct liquid or solid material. Note, this source focuses only on liquid ...
with a normal density range of 11 to 32 kg/m3. It is usually white and made of pre-expanded polystyrene beads. The manufacturing process for EPS conventionally begins with the creation of small polystyrene beads. Styrene monomers (and potentially other additives) are suspended in water, where they undergo free-radical polymerization. The polystyrene beads formed by this mechanism may have an average diameter of around 200 μm. The beads are then permeated with a "blowing agent", a material that enables the beads to be expanded. Pentane
Pentane is an organic compound with the chemical formula, formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomerism, structural isomers, or to a mixture of them: in the IUPAC nomenclature, h ...
is commonly used as the blowing agent. The beads are added to a continuously agitated reactor with the blowing agent, among other additives, and the blowing agent seeps into pores within each bead. The beads are then expanded using steam.
EPS is used for food containers, molded sheets for building insulation
Building insulation is material used in a building (specifically the building envelope) to reduce the flow of thermal energy. While the majority of insulation in buildings is for thermal insulation, thermal purposes, the term also applies to ...
, and packing material either as solid blocks formed to accommodate the item being protected or as loose-fill "peanuts" cushioning fragile items inside boxes. EPS also has been widely used in automotive and road safety applications such as motorcycle helmet
A motorcycle helmet is a type of helmet used by motorcycle riders. Motorcycle helmets contribute to motorcycle safety by protecting the rider's head in the event of an impact. They reduce the risk of head injury by 69% and the risk of death by 4 ...
s and road barriers on automobile race tracks.
A significant portion of all EPS products are manufactured through injection molding. Mold tools tend to be manufactured from steels (which can be hardened and plated), and aluminum alloys. The molds are controlled through a split via a channel system of gates and runners. EPS is colloquially called "styrofoam" in the Anglosphere
The Anglosphere, also known as the Anglo-American world, is a Western-led sphere of influence among the Anglophone countries. The core group of this sphere of influence comprises five developed countries that maintain close social, cultura ...
, a genericization of Dow Chemical's brand of extruded polystyrene.
EPS in building construction
Sheets of EPS are commonly packaged as rigid panels (common in Europe is a size of 100 cm x 50 cm, usually depending on an intended type of connection and glue techniques, it is, in fact, 99.5 cm x 49.5 cm or 98 cm x 48 cm; less common is 120 x 60 cm; size or in the United States). Common thicknesses are from 10 mm to 500 mm. Many customizations, additives, and thin additional external layers on one or both sides are often added to help with various properties. An example of this is lamination with cement board to form a structural insulated panel.Thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
is measured according to EN 12667. Typical values range from 0.032 to 0.038 W/(m⋅K) depending on the density of the EPS board. The value of 0.038 W/(m⋅K) was obtained at 15 kg/m3 while the value of 0.032 W/(m⋅K) was obtained at 40 kg/m3 according to the datasheet of K-710 from StyroChem Finland. Adding fillers (graphites, aluminum, or carbons) has recently allowed the thermal conductivity of EPS to reach around 0.030–0.034 W/(m⋅K) (as low as 0.029 W/(m⋅K)) and as such has a grey/black color which distinguishes it from standard EPS. Several EPS producers have produced a variety of these increased thermal resistance EPS usage for this product in the UK and EU.
Water vapor diffusion resistance (''μ'') of EPS is around 30–70.
ICC-ES ( International Code Council Evaluation Service) requires EPS boards used in building construction meet ASTM C578 requirements. One of these requirements is that the limiting oxygen index of EPS as measured by ASTM D2863 be greater than 24 volume %. Typical EPS has an oxygen index of around 18 volume %; thus, a flame retardant is added to styrene or polystyrene during the formation of EPS.
The boards containing a flame retardant when tested in a tunnel using test method UL 723 or ASTM E84 will have a flame spread index of less than 25 and a smoke-developed index of less than 450. ICC-ES requires the use of a 15-minute thermal barrier when EPS boards are used inside of a building.
According to the EPS-IA ICF organization, the typical density of EPS used for insulated concrete forms ( expanded polystyrene concrete) is . This is either Type II or Type IX EPS according to ASTM C578. EPS blocks or boards used in building construction are commonly cut using hot wires.
Extruded polystyrene (XPS)
 Extruded polystyrene foam (XPS) consists of closed cells. It offers improved surface roughness, higher stiffness and reduced thermal conductivity. The density range is about 28–34 kg/m3.
Extruded polystyrene material is also used in
Extruded polystyrene foam (XPS) consists of closed cells. It offers improved surface roughness, higher stiffness and reduced thermal conductivity. The density range is about 28–34 kg/m3.
Extruded polystyrene material is also used in craft
A craft or trade is a pastime or an occupation that requires particular skills and knowledge of skilled work. In a historical sense, particularly the Middle Ages and earlier, the term is usually applied to people occupied in small scale pr ...
s and model
A model is an informative representation of an object, person, or system. The term originally denoted the plans of a building in late 16th-century English, and derived via French and Italian ultimately from Latin , .
Models can be divided in ...
building, in particular architectural models. Because of the extrusion manufacturing process, XPS does not require facers to maintain its thermal or physical property performance. Thus, it makes a more uniform substitute for corrugated cardboard. Thermal conductivity varies between 0.029 and 0.039 W/(m·K) depending on bearing strength/density and the average value is ≈0.035 W/(m·K).
Water vapor diffusion resistance (μ) of XPS is around 80–250.
Commonly extruded polystyrene foam materials include:
* Styrofoam, also known as Blue Board, produced by DuPont
Dupont, DuPont, Du Pont, duPont, or du Pont may refer to:
People
* Dupont (surname) Dupont, also spelled as DuPont, duPont, Du Pont, or du Pont is a French surname meaning "of the bridge", historically indicating that the holder of the surname re ...
* Depron, a thin insulation sheet also used for model building
Water absorption of polystyrene foams
Although it is a closed-cell foam, both expanded and extruded polystyrene are not entirely waterproof or vapor proof. In expanded polystyrene there are interstitial gaps between the expanded closed-cell pellets that form an open network of channels between the bonded pellets, and this network of gaps can become filled with liquid water. If the water freezes into ice, it expands and can cause polystyrene pellets to break off from the foam. Extruded polystyrene is also permeable by water molecules and can not be considered a vapor barrier. Water-logging commonly occurs over a long period in polystyrene foams that are constantly exposed to high humidity or are continuously immersed in water, such as in hot tub covers, in floating docks, as supplemental flotation under boat seats, and for below-grade exterior building insulation constantly exposed to groundwater. Typically an exterior vapor barrier such as impermeable plastic sheeting or a sprayed-on coating is necessary to prevent saturation.Oriented polystyrene
Oriented polystyrene (OPS) is produced by stretching extruded PS film, improving visibility through the material by reducing haziness and increasing stiffness. This is often used in packaging where the manufacturer would like the consumer to see the enclosed product. Some benefits to OPS are that it is less expensive to produce than other clear plastics such aspolypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
(PP), polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in synthetic fibre, fibres for clothing, packaging, conta ...
(PET), and high-impact polystyrene (HIPS), and it is less hazy than HIPS or PP. The main disadvantage of OPS is that it is brittle, and will crack or tear easily.
Co-polymers
Ordinary ( homopolymeric) polystyrene has an excellent property profile about transparency, surface quality and stiffness. Its range of applications is further extended by copolymerization and other modifications ( blends e.g. with PC and syndiotactic polystyrene). Several copolymers are used based onstyrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
: The brittleness
A material is brittle if, when subjected to stress (physics), stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of h ...
of homopolymeric polystyrene is overcome by elastomer-modified styrene-butadiene copolymers. Copolymers of styrene and acrylonitrile ( SAN) are more resistant to thermal stress, heat and chemicals than homopolymers and are also transparent. Copolymers called ABS have similar properties and can be used at low temperatures, but they are opaque.
Styrene-butane co-polymers
Styrene-butane co-polymers can be produced with a low butene content. Styrene-butane co-polymers include PS-I and SBC (see below), both co-polymers are impact resistant. PS-I is prepared by graft co-polymerization, SBC by anionic block co-polymerization, which makes it transparent in case of appropriate block size. If styrene-butane co-polymer has a high butylene content, styrene-butadiene rubber (SBR) is formed. The impact strength of styrene-butadiene co-polymers is based on phase separation, polystyrene and poly-butane are not soluble in each other (see Flory–Huggins solution theory). Co-polymerization creates a boundary layer without complete mixing. The butadiene fractions (the "rubber phase") assemble to form particles embedded in a polystyrene matrix. A decisive factor for the improved impact strength of styrene-butadiene copolymers is their higher absorption capacity for deformation work. Without applied force, the rubber phase initially behaves like a filler. Under tensile stress, crazes (microcracks) are formed, which spread to the rubber particles. The energy of the propagating crack is then transferred to the rubber particles along its path. A large number of cracks give the originally rigid material a laminated structure. The formation of each lamella contributes to the consumption of energy and thus to an increase in elongation at break. Polystyrene homo-polymers deform when a force is applied until they break. Styrene-butane co-polymers do not break at this point, but begin to flow, solidify to tensile strength and only break at much higher elongation. With a high proportion of polybutadiene, the effect of the two phases is reversed. Styrene-butadiene rubber behaves like an elastomer but can be processed like a thermoplastic.Impact-resistant polystyrene (PS-I)
PS-I (''impact resistant polystyrene'') consists of a continuous polystyrene matrix and a rubber phase dispersed therein. It is produced by polymerization of styrene in the presence of polybutadiene dissolved (in styrene). Polymerization takes place simultaneously in two ways: * Graft copolymerization: The growing polystyrene chain reacts with adouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
of the polybutadiene. As a result, several polystyrene chains are attached to one polybutadiene.
** S represents in the figure the styrene repeat unit
** B the butadiene repeat unit. However, the middle block often does not consist of such depicted butane homo-polymer but of a styrene-butadiene co-polymer:
:::SSSSSSSSSSSSSSSSSSSBBSBBSBSBBBBSBSSBBBSBSSSSSSSSSSSSSSSSSSSSSSSSSSSSSS
By using a statistical copolymer at this position, the polymer becomes less susceptible to cross-linking and flows better in the melt. For the production of SBS, the first styrene is homopolymerized via anionic copolymerization. Typically, an organometallic compound such as butyllithium is used as a catalyst. Butadiene is then added and after styrene again its polymerization. The catalyst remains active during the whole process (for which the used chemicals must be of high purity). The molecular weight distribution of the polymers is very low ( polydispersity in the range of 1.05, the individual chains have thus very similar lengths). The length of the individual blocks can be adjusted by the ratio of catalyst to monomer. The size of the rubber sections, in turn, depends on the block length. The production of small structures (smaller than the wavelength of the light) ensure transparency. In contrast to PS-I, however, the block copolymer does not form any particles but has a lamellar structure.
Styrene-butadiene rubber
Styrene-butadiene rubber (SBR) is produced like PS-I by graft copolymerization, but with a lower styrene content. Styrene-butadiene rubber thus consists of a rubber matrix with a polystyrene phase dispersed therein. Unlike PS-I and SBC, it is not athermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
, but an elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
. Within the rubber phase, the polystyrene phase is assembled into domains. This causes physical cross-linking on a microscopic level. When the material is heated above the glass transition point, the domains disintegrate, the cross-linking is temporarily suspended and the material can be processed like a thermoplastic.
Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene (ABS) is a material that is stronger than pure polystyrene.Others
SMA is a copolymer with maleic anhydride. Styrene can be copolymerized with other monomers; for example, divinylbenzene can be used for cross-linking the polystyrene chains to give the polymer used in solid phase peptide synthesis. Styrene-acrylonitrile resin (SAN) has a greater thermal resistance than pure styrene.Environmental issues
Production
Polystyrene foams are produced using blowing agents that form bubbles and expand the foam. In expanded polystyrene, these are usually hydrocarbons such aspentane
Pentane is an organic compound with the chemical formula, formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomerism, structural isomers, or to a mixture of them: in the IUPAC nomenclature, h ...
, which may pose a flammability hazard in manufacturing or storage of newly manufactured material, but have relatively mild environmental impact. Extruded polystyrene is usually made with hydrofluorocarbon
Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air condit ...
s ( HFC-134a), which have global warming potentials of approximately 1000–1300 times that of carbon dioxide. In Europe, where HFC-134a was banned since beginning 2022, XPS is produced using carbon dioxide as blowing agent, achieving an ODP (Ozone depletion potential) of 0 and a GWP (Global warming potential) below 5.Packaging, particularly expanded polystyrene, is a contributor of microplastics
Microplastics are "synthetic solid particles or polymeric matrices, with regular or irregular shape and with size ranging from 1 μm to 5 mm, of either primary or secondary manufacturing origin, which are insoluble in water." Microplastics a ...
from both land and maritime activities.
Environmental degradation
Polystyrene is not biodegradeable but it is susceptible to photo-oxidation. For this reason commercial products contain light stabilizers.Litter
 Animals do not recognize polystyrene foam as an artificial material and may even mistake it for food.
Polystyrene foam blows in the wind and floats on water due to its low specific gravity. It can have serious effects on the health of birds and marine animals that swallow significant quantities. Juvenile rainbow trout exposed to polystyrene fragments show toxic effects in the form of substantial histomorphometrical changes.
Animals do not recognize polystyrene foam as an artificial material and may even mistake it for food.
Polystyrene foam blows in the wind and floats on water due to its low specific gravity. It can have serious effects on the health of birds and marine animals that swallow significant quantities. Juvenile rainbow trout exposed to polystyrene fragments show toxic effects in the form of substantial histomorphometrical changes.
Reducing
Restricting the use of foamed polystyrene takeout food packaging is a priority of many solid waste environmental organisations. Efforts have been made to find alternatives to polystyrene, especially foam in restaurant settings. The original impetus was to eliminatechlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volat ...
s (CFC), which was a former component of foam.
United States
In 1987,Berkeley, California
Berkeley ( ) is a city on the eastern shore of San Francisco Bay in northern Alameda County, California, United States. It is named after the 18th-century Anglo-Irish bishop and philosopher George Berkeley. It borders the cities of Oakland, Cali ...
, banned CFC food containers. The following year, Suffolk County, New York
Suffolk County ( ) is the easternmost county in the U.S. state of New York, constituting the eastern two-thirds of Long Island. It is bordered to its west by Nassau County, to its east by Gardiners Bay and the open Atlantic Ocean, to its no ...
, became the first U.S. jurisdiction to ban polystyrene in general. However, legal challenges by the Society of the Plastics Industry kept the ban from going into effect until at last it was delayed when the Republican and Conservative parties gained the majority of the county legislature. In the meantime, Berkeley became the first city to ban all foam food containers. As of 2006, about one hundred localities in the United States, including Portland, Oregon
Portland ( ) is the List of cities in Oregon, most populous city in the U.S. state of Oregon, located in the Pacific Northwest region. Situated close to northwest Oregon at the confluence of the Willamette River, Willamette and Columbia River, ...
, and San Francisco
San Francisco, officially the City and County of San Francisco, is a commercial, Financial District, San Francisco, financial, and Culture of San Francisco, cultural center of Northern California. With a population of 827,526 residents as of ...
had some sort of ban on polystyrene foam in restaurants. For instance, in 2007 Oakland, California
Oakland is a city in the East Bay region of the San Francisco Bay Area in the U.S. state of California. It is the county seat and most populous city in Alameda County, California, Alameda County, with a population of 440,646 in 2020. A major We ...
, required restaurants to switch to disposable food containers that would biodegrade if added to food compost. In 2013, San Jose became reportedly the largest city in the country to ban polystyrene foam food containers. Some communities have implemented wide polystyrene bans, such as Freeport, Maine, which did so in 1990. In 1988, the first U.S. ban of general polystyrene foam was enacted in Berkeley, California.
On 1 July 2015, New York City
New York, often called New York City (NYC), is the most populous city in the United States, located at the southern tip of New York State on one of the world's largest natural harbors. The city comprises five boroughs, each coextensive w ...
became the largest city in the United States to attempt to prohibit the sale, possession, and distribution of single-use polystyrene foam (the initial decision was overturned on appeal). In San Francisco, supervisors approved the toughest ban on "Styrofoam" (EPS) in the US which went into effect 1 January 2017. The city's Department of the Environment can make exceptions for certain uses like shipping medicines at prescribed temperatures.
The U.S. Green Restaurant Association does not allow polystyrene foam to be used as part of its certification standard. Several green leaders, including the Dutch Ministry of the Environment, advise people to reduce their environmental harm by using reusable coffee cups.
In March 2019, Maryland banned polystyrene foam food containers and became the first state in the country to pass a food container foam ban through the state legislature. Maine was the first state to officially get a foam food container ban onto the books. In May 2019, Maryland Governor Hogan allowed the foam ban (House Bill 109) to become law without a signature making Maryland the second state to have a food container foam ban on the books, but is the first one to take effect on 1 July 2020.
In September 2020, the New Jersey state legislature voted to ban disposable foam food containers and cups made of polystyrene foam.
Outside the United States
China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
banned expanded polystyrene takeout/takeaway containers and tableware around 1999. However, compliance has been a problem and, in 2013, the Chinese plastics industry was lobbying for the ban's repeal.
India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
and Taiwan
Taiwan, officially the Republic of China (ROC), is a country in East Asia. The main geography of Taiwan, island of Taiwan, also known as ''Formosa'', lies between the East China Sea, East and South China Seas in the northwestern Pacific Ocea ...
also banned polystyrene-foam food-service ware before 2007.
The government of Zimbabwe
file:Zimbabwe, relief map.jpg, upright=1.22, Zimbabwe, relief map
Zimbabwe, officially the Republic of Zimbabwe, is a landlocked country in Southeast Africa, between the Zambezi and Limpopo Rivers, bordered by South Africa to the south, Bots ...
, through its Environmental Management Agency (EMA), banned polystyrene containers (popularly called 'kaylite' in the country), under Statutory Instrument 84 of 2012 (Plastic Packaging and Plastic Bottles) (Amendment) Regulations, 2012 (No 1.)
The city of Vancouver
Vancouver is a major city in Western Canada, located in the Lower Mainland region of British Columbia. As the List of cities in British Columbia, most populous city in the province, the 2021 Canadian census recorded 662,248 people in the cit ...
, Canada, has announced its Zero Waste 2040 plan in 2018. The city will introduce bylaw amendments to prohibit business license holders from serving prepared food in polystyrene foam cups and take-out containers, beginning 1 June 2019.
In 2019, the European Union voted to ban expanded polystyrene food packaging and cups, with the law officially going into effect in 2021.
Fiji
Fiji, officially the Republic of Fiji, is an island country in Melanesia, part of Oceania in the South Pacific Ocean. It lies about north-northeast of New Zealand. Fiji consists of an archipelago of more than 330 islands—of which about ...
passed the Environmental Management Bill in December 2020. Imports of polystyrene products were banned in January 2021.
Recycling
Upcycling
A March 2022 joint study by scientists Sewon Oh and Erin Stache at Cornell University in Ithaca, New York found a new processing method of upcycling polystyrene to benzoic acid. The process involved irradiation of polystyrene with iron chloride and acetone under white light and oxygen for 20 hours. The scientists also demonstrated a similar scalable commercial process of upcycling polystyrene into valuable small-molecules (like benzoic acid) taking just a few hours.Incineration
If polystyrene is properly incinerated at high temperatures (up to 1000 °CBASF Technische Information TI 0/2-810d 81677 Juni 1989, Verwertungs- und Beseitigungsverfaren gebrauchter Schaumstoff-Verpackungen aus Styropor®) and with plenty of air (14 m3/kg), the chemicals generated are water, carbon dioxide, and possibly small amounts of residual halogen-compounds from flame-retardants. If only incomplete incineration is done, there will also be leftover carbon soot and a complex mixture of volatile compounds.Polystyrene Foam Burning Danger. Newton.dep.anl.gov. Retrieved 25 December 2011. Q and A page with an partially incorrect information. According to the American Chemistry Council, when polystyrene is incinerated in modern facilities, the final volume is 1% of the starting volume; most of the polystyrene is converted into carbon dioxide, water vapor, and heat. Because of the amount of heat released, it is sometimes used as a power source for steam or electricity generation. When polystyrene was burned at temperatures of 800–900 °C (the typical range of a modern incinerator), the products of combustion consisted of "a complex mixture of polycyclic aromatic hydrocarbons (PAHs) from alkyl benzenes to benzoperylene. Over 90 different compounds were identified in combustion effluents from polystyrene." The American National Bureau of Standards Center for Fire Research found 57 chemical by-products released during the combustion of expanded polystyrene (EPS) foam.
Safety
Health
The American Chemistry Council, formerly known as the Chemical Manufacturers' Association, wrote in 2011: From 1999 to 2002, a comprehensive review of the potential health risks associated with exposure to styrene was conducted by a 12-member international expert panel selected by the Harvard Center for Risk Assessment. The scientists had expertise in toxicology, epidemiology, medicine, risk analysis, pharmacokinetics, and exposure assessment. The Harvard study reported that styrene is naturally present in trace quantities in foods such as strawberries, beef, and spices, and is naturally produced in the processing of foods such as wine and cheese. The study also reviewed all the published data on the quantity of styrene contributing to the diet due to migration of food packaging and disposable food contact articles, and concluded that risk to the general public from exposure to styrene from foods or food-contact applications (such as polystyrene packaging and foodservice containers) was at levels too low to produce adverse effects. Polystyrene is commonly used in containers for food and drinks. The styrene monomer (from which polystyrene is made) is a cancer suspect agent. Styrene is "generally found in such low levels in consumer products that risks aren't substantial". Polystyrene which is used for food contact may not contain more than 1% (0.5% for fatty foods) of styrene by weight. Styrene oligomers in polystyrene containers used for food packaging have been found to migrate into the food. Another Japanese study conducted on wild-type and Aryl hydrocarbon receptor, AhR-null mice found that the styrene trimer, which the authors detected in cooked polystyrene container-packed instant foods, may increase thyroid hormone levels. Whether polystyrene can be microwaved with food is controversial. Some containers may be safely used in a microwave, but only if labeled as such. Some sources suggest that foods containing carotene (vitamin A) or cooking oils must be avoided. Because of the pervasive use of polystyrene, these serious health related issues remain topical.Fire hazards
Like other organic compounds, polystyrene is flammable. Polystyrene is classified according to DIN4102 A1, DIN4102 as a "B3" product, meaning highly flammable or "Easily Ignited". As a consequence, although it is an efficient insulator at low temperatures, its use is prohibited in any exposed installations in building construction if the material is not flame retardant, flame-retardant. It must be concealed behind drywall, sheet metal, or concrete. Foamed polystyrene plastic materials have been accidentally ignited and caused huge fires and losses of life, for example at the Düsseldorf Airport fire, Düsseldorf International Airport and in the 1996 Channel Tunnel fire, Channel Tunnel (where polystyrene was inside a railway carriage that caught fire).See also
* Styrofoam * Foam food container * Bioplastic * Geofoam * Structural insulated panel * Polystyrene sulfonate * Shrinky Dinks * Insulating concrete form * Foamcore * Phase-out of polystyrene foamReferences
Sources
Bibliography
*External links
Polystyrene Composition
– The University of Southern Mississippi
SPI resin identification code
– Society of the Plastics Industry
Polystyrene: Local Ordinances
– Californians Against Waste
Take a Closer Look at Today's Polystyrene Packaging
(brochure by the industry group American Chemistry Council, arguing that the material is "safe, affordable and environmentally responsible") * {{Authority control Insulators Building insulation materials Organic polymers Packaging materials Food packaging Thermoplastics Commodity chemicals Vinyl polymers