enolate on:
[Wikipedia]
[Google]
[Amazon]
 In
In
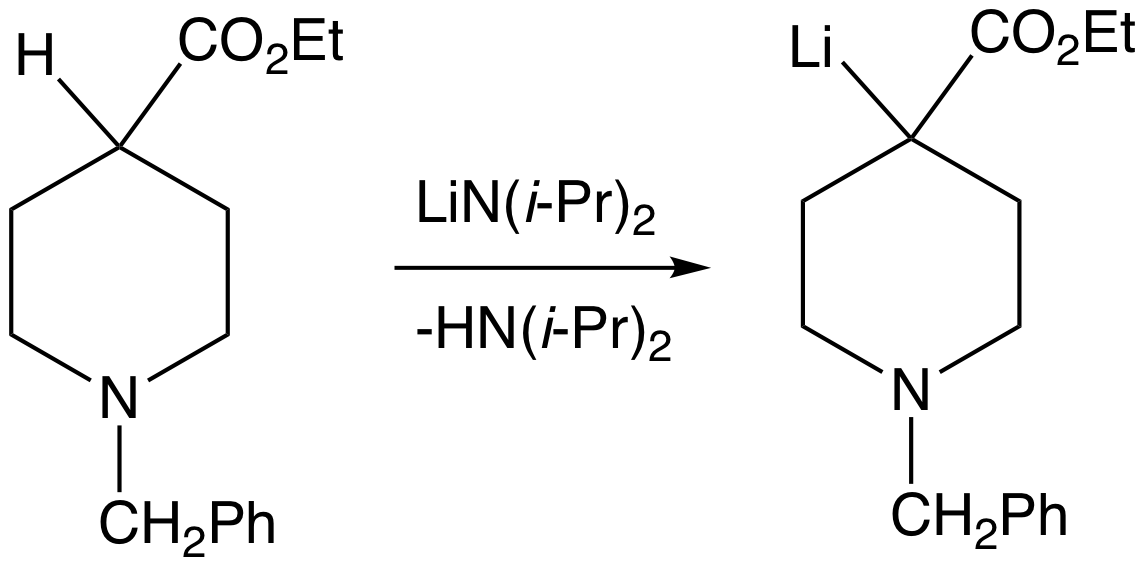 The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of
The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of
 For
For
 The stereoselective formation of enolates has been rationalized with the Ireland model, although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are
The stereoselective formation of enolates has been rationalized with the Ireland model, although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are
 The trisubstituted enolate is considered the kinetic enolate, while the tetrasubstituted enolate is considered the thermodynamic enolate. The alpha hydrogen deprotonated to form the kinetic enolate is less hindered, and therefore deprotonated more quickly. In general, tetrasubstituted olefins are more stable than trisubstituted olefins due to hyperconjugative stabilization. The ratio of enolate regioisomers is heavily influenced by the choice of base. For the above example, kinetic control may be established with LDA at −78 °C, giving 99:1 selectivity of kinetic: thermodynamic enolate, while thermodynamic control may be established with triphenylmethyllithium at
The trisubstituted enolate is considered the kinetic enolate, while the tetrasubstituted enolate is considered the thermodynamic enolate. The alpha hydrogen deprotonated to form the kinetic enolate is less hindered, and therefore deprotonated more quickly. In general, tetrasubstituted olefins are more stable than trisubstituted olefins due to hyperconjugative stabilization. The ratio of enolate regioisomers is heavily influenced by the choice of base. For the above example, kinetic control may be established with LDA at −78 °C, giving 99:1 selectivity of kinetic: thermodynamic enolate, while thermodynamic control may be established with triphenylmethyllithium at

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, enolates are organic anions derived from the deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
of carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
() compounds. Rarely isolated, they are widely used as reagents
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
in the synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
of organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s.
Bonding and structure
Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both analkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
and a carbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
.
Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates.
Preparation
Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from usinglithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
(LDA).
Often, as in conventional Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β- diketone. It is named ...
s, Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia () ...
s, and aldol condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration t ...
s, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concentrations, but they still undergo reactions with electrophiles. Many factors affect the behavior of enolates, especially the solvent, additives (e.g. diamines), and the countercation (Li+ vs Na+, etc.). For unsymmetrical ketones, methods exist to control the regiochemistry of the deprotonation.
 The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of
The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of phenylacetone
Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono- substituted benzene derivative, consisting of an acetone att ...
, deprotonation can produce two different enolates. LDA has been shown to deprotonate the methyl group, which is the kinetic course of the deprotonation. To ensure the production of the kinetic product, a slight excess (1.1 equiv) of lithium diisopropylamide is used, and the ketone is added to the base at −78 °C. Because the ketone is quickly and quantitatively converted to the enolate and base is present in excess at all times, the ketone is unable to act as a proton shuttle to catalyze the gradual formation of the thermodynamic product. A weaker base such as an alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
, which reversibly deprotonates the substrate, affords the more thermodynamically stable benzylic enolate.
Enolates can be trapped by acylation
In chemistry, acylation is a broad class of chemical reactions in which an acyl group () is added to a substrate. The compound providing the acyl group is called the acylating agent. The substrate to be acylated and the product include the foll ...
and silylation, which occur at oxygen. Silyl enol ethers are common reagents in organic synthesis as illustrated by the Mukaiyama aldol reaction:
Role of Lewis acids on enolate formation
In addition to the use of strong bases, enolates can be generated using aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
and a weak base ("soft conditions"):
deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
to occur, the stereoelectronic requirement is that the alpha-C-H sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
must be able to overlap with the pi* orbital of the carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
:
Geometry
Extensive studies have been performed on the formation of enolates. It is possible to control the geometry of the enolate: For ketones, most enolization conditions give ''Z'' enolates. Forester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, most enolization conditions give ''E'' enolates. The addition of HMPA is known to reverse the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
of deprotonation.
monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
ic or oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
ic in nature; nonetheless, the Ireland model remains a useful tool for understanding enolates.
In the Ireland model, the deprotonation is assumed to proceed by a six-membered or cyclic monomeric transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
. The larger of the two substituents on the electrophile (in the case above, methyl is larger than proton) adopts an equatorial disposition in the favored transition state, leading to a preference for E enolates. The model clearly fails in many cases; for example, if the solvent mixture is changed from THF to 23% HMPA-THF (as seen above), the enolate geometry is reversed, which is inconsistent with this model and its cyclic transition state.
Regiochemistry of enolate formation
If an unsymmetrical ketone is subjected to base, it has the potential to form two regioisomeric enolates (ignoring enolate geometry). For example:room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
, giving 10:90 selectivity.
In general, kinetic enolates are favored by cold temperatures, conditions that give relatively ionic metal–oxygen bonding, and rapid deprotonation using a slight excess of a strong, sterically hindered base. The large base only deprotonates the more accessible hydrogen, and the low temperatures and excess base help avoid equilibration to the more stable alternate enolate after initial enolate formation. Thermodynamic enolates are favored by longer equilibration times at higher temperatures, conditions that give relatively covalent metal–oxygen bonding, and use of a slight sub-stoichiometric amount of strong base. By using insufficient base to deprotonate all of the carbonyl molecules, the enolates and carbonyls can exchange protons with each other and equilibrate to their more stable isomer. Using various metals and solvents can provide control over the amount of ionic character in the metal–oxygen bond.
Reactions
As powerful nucleophiles, enolates react with a variety of electrophiles. The stereoselectivity and regioselectivity is influenced by additives, solvent,counterion
160px, cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.
In chemistry, a counterion (sometimes written as "counter ...
s, etc. When the electrophiles are alkyl halides, a classic problem arises: O-alkylation vs C-alkylation. Controlling this selectivity has drawn much attention. The negative charge in enolates is concentrated on the oxygen, but that center is also highly solvated, which leads to C-alkylation.
Other important electrophiles are aldehydes/ketones and Michael acceptors.
:
Synthesis of enones using regiospecific enolate formation and masked functionality
Regiospecific formation is the controlled enolate formation by the specific deprotonation at one of the α-carbons of the ketone starting molecule. This provides one of the best understood synthetic strategies to introduce chemical complexity innatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
and total syntheses. A prominent example of its use is in the total synthesis of progesterone illustrated in Figure "Regiospecific enolate formation in the total synthesis of progesterone".
When ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
are treated with base, enolates can be formed by deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
at either α-carbon. The selectivity is determined by both the steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
and electronic effects on the α-carbons as well as the precise base used (see figure ""Masked functionality" for regiospecific enolate formation" for an example of this). Enolate formation will be thermodynamically favoured at the most acidic proton which depends on the electronic stabilization of the resulting anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
. However, the selectivity can be reversed by sterically hindering the thermodynamic product and therefore kinetically favouring deprotonation at the other α-carbon centre. Traditional methods for regioselective enolate formation use either electronic activating groups (e.g. aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
) or steric blocking groups (e.g. 1,2-ethanedithiol protected ketone).
An enone can also serve as a precursor for regiospecific formation of an enolate, here the enone is a "masked functionality" for the enolate. This process is first described by Gilbert Stork who is best known for his contributions to the study of selective enolate formation methods in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. Reacting an enone with lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
metal generates the enolate at the α-carbon of the enone. The enolate product can either be trapped or alkylated. By using "masked functionality", it is possible to produce enolates that are not accessible by traditional methods.
The "masked functionality" approach to regiospecific enolate formation has been widely used in the total synthesis of natural products. For example, in the total synthesis of the steroid hormone progesterone
Progesterone (; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the ma ...
, Stork and co-workers used the "masked functionality" to stereospecifically construct one of the quaternary
The Quaternary ( ) is the current and most recent of the three periods of the Cenozoic Era in the geologic time scale of the International Commission on Stratigraphy (ICS), as well as the current and most recent of the twelve periods of the ...
carbons in the molecule.
Aza enolates
Aza enolates (also known as imine anions, enamides, metallated Schiff bases, and metalloenamines) are nitrogen analogous to enolates. When imines get treated with strong bases such as LDA, highly nucleophilic aza enolates are generated. The major benefit of using aza enolates is that they don't undergo self-condensation (i.e.aldol reaction
The aldol reaction (aldol addition) is a Chemical reaction, reaction in organic chemistry that combines two Carbonyl group, carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might invol ...
for aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
) in a basic or neutral solution, but rather they favor alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
on the alpha-carbon. This is mainly because imines
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
contain carbon-nitrogen double bonds unlike aldehydes, which contain oxygen-carbon double bonds. Since oxygen is more electronegative than nitrogen, it withdraws more electron density from the carbonyl carbon, inducing a greater partially positive charge on the carbon. Therefore, with more electrophilic carbon, aldehydes allow for better nucleophilic addition to the carbon on the carbon-oxygen double bond.
On the other hand, imine has less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
nitrogen which induces a weaker partially positive charge on the carbonyl-carbon. As a result, while imines can still react with organolithiums, they don't react with other nucleophiles (including aza enolates) to undergo nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
s.
Instead, aza enolates react similarly to enolates, forming SN2 alkylated products. Through nitrogen lone pair conjugation, β-carbon becomes a nucleophilic site, permitting aza enolates to undergo alkylation reactions. Thus, aza enolates can react with numerous electrophiles like epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
s and alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
to form a new carbon-carbon bond on β-carbon.
Two potential reaction mechanisms are shown below:
Since epoxide is a three-membered ring molecule, it has a high degree of ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles ar ...
. Although the carbons in the ring system are tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
, preferring 109.5 degrees between each atom, epoxide strains the ring angles into 60 degrees. To counter this effect, the nucleophilic aza enolates easily react with epoxides to reduce their ring strains.
Besides reacting with epoxides, aza enolates can also react with alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
(or allyl halides as depicted above) to form a new carbon-carbon sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
. This reaction is one of the key steps in the synthesis of the male aggression pheromone, Oulema melanopus. Aza enolate is generated by LDA reacting with pivaldehyde, which then reacts with an alkyl halide to form an Oulema melanopus intermediate.
Aza enolates can also be formed with Grignard reagents and react with other soft electrophiles, including Michael receptors.
See also
* Nitrile anionReferences
{{Authority control Alkene derivatives Reactive intermediates Organic reactions de:Tautomerie#Keto-Enol-Tautomerie