Dithiobenzoate on:
[Wikipedia]
[Google]
[Amazon]
Dithiobenzoic acid is the
organosulfur compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulf ...
with the formula C6H5CS2H. It is a dithiocarboxylic acid, an analogue of benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, w ...
, but more acidic and deeply colored.
Synthesis and reactions
It can be prepared by sulfiding benzal chloride: :C6H5CCl3 + 4 KSH → C6H5CS2K + 3 KCl + 2 H2S :C6H5CS2K + H+ → C6H5CS2H + K+ It also arises by the reaction of the Grignard reagentphenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It i ...
with carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical ...
, followed by acidification:
:C6H5MgBr + CS2 → C6H5CS2MgBr
:C6H5CS2MgBr + HCl → C6H5CS2H + MgBrCl
It is about 100x more acidic than benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, w ...
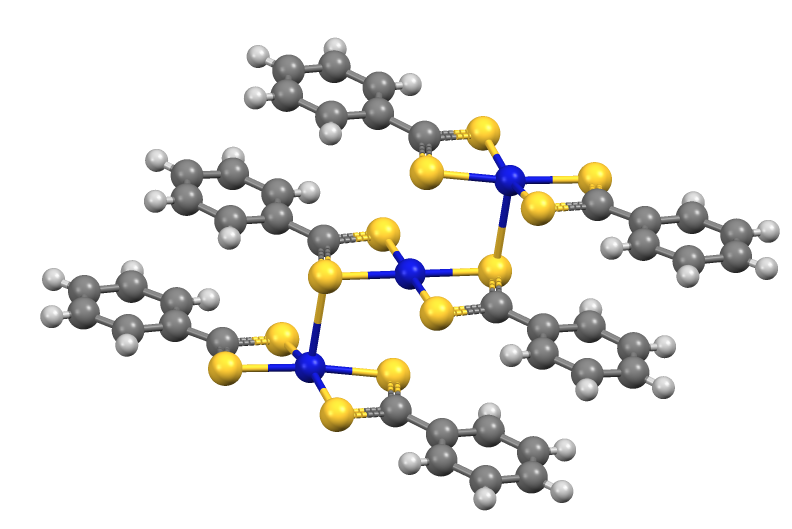
. Its conjugate base, dithiobenzoate, undergoes S-alkylation to give dithiocarboxylate esters. Similarly, dithiobenzoate reacts with "soft" metal salts to give complexes, e.g. Fe(S2CC6H5)3 and Ni(S2CC6H5)2.
Chlorination of dithiobenzoic acid gives the thioacyl chloride C6H5C(S)Cl.

References