Di-π-methane Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
In
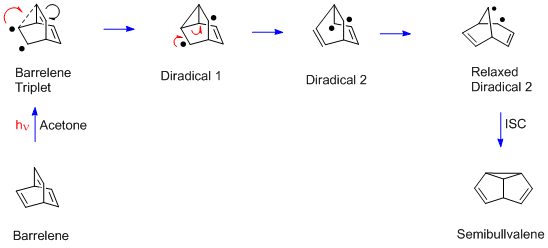 The barrelene rearrangement is more complex than the Mariano and Pratt examples since there are two ''sp''3-hybridized carbons. Each bridgehead carbon has three (ethylenic) π bonds, and any two can undergo the diπ-methane rearrangement. Moreover, unlike the acyclic Mariano and Pratt dienes, the barrelene reaction requires a triplet excited state. Thus
The barrelene rearrangement is more complex than the Mariano and Pratt examples since there are two ''sp''3-hybridized carbons. Each bridgehead carbon has three (ethylenic) π bonds, and any two can undergo the diπ-methane rearrangement. Moreover, unlike the acyclic Mariano and Pratt dienes, the barrelene reaction requires a triplet excited state. Thus
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the di-π-methane rearrangement is the photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible (400–750&nb ...
rearrangement of a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
that contains two π-systems separated by a saturated carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom. In the aliphatic case, this molecules is a 1,4-diene; in the aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
case, an allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
-substituted arene
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
. The reaction forms (respectively) an ene- or aryl-substituted cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
. Formally, it amounts to a 1,2 shift of one ene group (in the diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
) or the aryl group (in the allyl-aromatic analog), followed by bond formation between the lateral carbons of the non-migrating moiety:
Discovery
This rearrangement was originally encountered in thephotolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
of barrelene to give semibullvalene. Once the mechanism was recognized as general by Howard Zimmerman
Howard E. Zimmerman (July 5, 1926 – February 12, 2012) was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemis ...
in 1967, it was clear that the structural requirement was two π groups attached to an ''sp''3-hybridized carbon, and then a variety of further examples was obtained.
Notable examples
One example was the photolysis of Mariano's compound, 3,3dimethyl-1,1,5,5tetraphenyl-1,4pentadiene. In thissymmetric
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is invariant under some transformations ...
diene, the active π bonds are conjugated to arenes, which does not inhibit the reaction.
Another was the asymmetric Pratt diene. Pratt's diene demonstrates that the reaction preferentially cyclopropanates aryl substituents, because the reaction pathway preserves the resonant
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
stabilization of a benzhydryl
The benzhydryl compounds are a group of organic compounds whose parent structure, parent structural formula, structures include diphenylmethane (which is two benzene rings connected by a single substituent#Methane substituents, methane), with any ...
ic radical intermediate.
 The barrelene rearrangement is more complex than the Mariano and Pratt examples since there are two ''sp''3-hybridized carbons. Each bridgehead carbon has three (ethylenic) π bonds, and any two can undergo the diπ-methane rearrangement. Moreover, unlike the acyclic Mariano and Pratt dienes, the barrelene reaction requires a triplet excited state. Thus
The barrelene rearrangement is more complex than the Mariano and Pratt examples since there are two ''sp''3-hybridized carbons. Each bridgehead carbon has three (ethylenic) π bonds, and any two can undergo the diπ-methane rearrangement. Moreover, unlike the acyclic Mariano and Pratt dienes, the barrelene reaction requires a triplet excited state. Thus acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
is used in the barrelene reaction; acetone captures the light and then delivers triplet excitation to the barrelene reactant. In the final step of the rearrangement there is a spin flip, to provide paired electrons and a new σ bond.
As excited-state probe
The dependence of the di-π-methane rearrangement on the multiplicity of the excited state arises from the free-rotor effect.{{cite journal , last1 = Zimmerman , first1 = H. E. , last2 = Schissel , first2 = D. N , year = 1986 , title = Di-{{pi-methane Rearrangement of Highly Sterically Congested Molecules: Inhibition of Free Rotor Energy Dissipation. Mechanistic and Exploratory Organic Photochemistry , journal = J. Org. Chem. , volume = 51 , pages = 196–207 , doi=10.1021/jo00352a013 Triplet 1,4-dienes freely undergo ''cis''-''trans'' interconversion of diene double bonds (i.e. free rotation). In acyclic dienes, this free rotation leads to diradical reconnection, short-circuiting the di-π-methane process. Singlet excited states do not rotate and may thus undergo the di-π-methane mechanism. For cyclic dienes, as in the barrelene example, the ring structure can prevent free-rotatory dissipation, and may in fact require bond rotation to complete the rearrangement.References