Dess–Martin periodinane on:
[Wikipedia]
[Google]
[Amazon]
Dess–Martin periodinane (DMP) is a 
 The classic method presented by R. K. Boeckman and J. J. Mullins involved heating a solution of
The classic method presented by R. K. Boeckman and J. J. Mullins involved heating a solution of 
 Schreiber and coworkers have shown that water increases the rate of the oxidation reaction. Dess and Martin had originally observed that the oxidation of ethanol was increased when there was an extra equivalent of ethanol. It is believed that the rate of dissociation of the final acetate ligand from the iodine is increased, because of the
Schreiber and coworkers have shown that water increases the rate of the oxidation reaction. Dess and Martin had originally observed that the oxidation of ethanol was increased when there was an extra equivalent of ethanol. It is believed that the rate of dissociation of the final acetate ligand from the iodine is increased, because of the 


chemical reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
used in the Dess–Martin oxidation
The Dess–Martin oxidation is an organic reaction for the redox, oxidation of primary Alcohol (chemistry), alcohols to aldehydes and secondary alcohols to ketones using Dess–Martin periodinane.
It is named after the American chemists Daniel Be ...
, oxidizing primary alcohols to aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s and secondary alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s to ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s. This periodinane Periodinanes also known as lambda, λ5-iodanes are organoiodine compounds with iodine in the +5 oxidation state. These compounds are described as hypervalency, hypervalent because the iodine center has more than 8 valence electrons.
Periodinane com ...
has several advantages over chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
- and DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
-based oxidants that include milder conditions (room temperature, neutral pH), shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. However, use on an industrial scale is made difficult by its cost and its potentially explosive nature. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin
James Cullen Martin (January 14, 1928 – April 20, 1999) was an American chemist. Known in the field as "J.C.", he specialized in physical organic chemistry with an emphasis on main group element chemistry.
Martin received his undergraduat ...
who developed the reagent in 1983. It is based on IBX
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant su ...
, but due to the acetate groups attached to the central iodine atom, DMP is much more reactive than IBX and is much more soluble in organic solvents.
:
Preparation
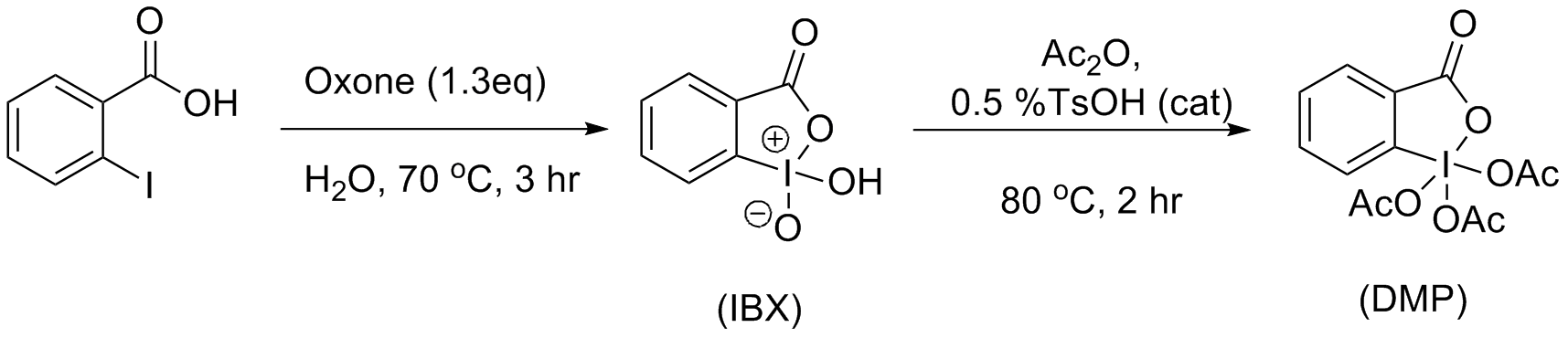
The most friendly synthesis of IBX has been determined to be treating 2-iodobenzoic acid withoxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
in water, at elevated temperatures for 3 hours. IBX is then acylated using Ireland and Liu’s
modifications from the original procedure. These modifications allowed for higher yields and a simplified work up procedure. The resulted solids can be obtained via filtration and washing with ether. Ireland and Liu used a catalytic amount of tosylic acid, which allowed the reaction to complete in less than 2 hours (compared to the classic synthesis, utilizing 24 hours) and in yields exceeding 90%.
: The classic method presented by R. K. Boeckman and J. J. Mullins involved heating a solution of
The classic method presented by R. K. Boeckman and J. J. Mullins involved heating a solution of potassium bromate
Potassium bromate () is a bromate of potassium and takes the form of white crystals or powder. It is a strong oxidizing agent.
Preparation and structure
Potassium bromate is produced when bromine is passed through a hot solution of potassium hyd ...
, sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, 2-iodobenzoic acid to afford IBX (1-hydroxy-1,2-benziodoxol-3(1H)-one 1-oxide, 2-iodoxybenzoic acid
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant su ...
). IBX was then acylated using acetic acid and acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
.
:Structure
DMP has square pyramidal geometry with 4 heteroatoms in basal positions and one apical phenyl group.Oxidation mechanism
Dess–Martin periodinane is mainly used as anoxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
for complex, sensitive and multifunctional alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s. One of the reasons for its effectiveness is its high selectivity towards complexation of the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group, which allows alcohols to rapidly perform ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
exchange; the first step in the oxidation reaction.
Proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the stru ...
has indicated that using one equivalent of alcohol forms the intermediate diacetoxyalkoxyperiodinane. The acetate then acts as a base to deprotonate the α-H from the alcohol to afford the carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
compound, iodinane, and acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
.
When a diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting gro ...
or more than one equivalent of alcohol is used, acetoxydialkoxyperiodinane is formed instead. Due to the labile
Lability refers to the degree that something is likely to undergo change. It is the opposite ( antonym) of stability.
Biochemistry
In reference to biochemistry, this is an important concept as far as kinetics is concerned in metalloprotein ...
nature of this particular periodinane, oxidation occurs much faster.
: Schreiber and coworkers have shown that water increases the rate of the oxidation reaction. Dess and Martin had originally observed that the oxidation of ethanol was increased when there was an extra equivalent of ethanol. It is believed that the rate of dissociation of the final acetate ligand from the iodine is increased, because of the
Schreiber and coworkers have shown that water increases the rate of the oxidation reaction. Dess and Martin had originally observed that the oxidation of ethanol was increased when there was an extra equivalent of ethanol. It is believed that the rate of dissociation of the final acetate ligand from the iodine is increased, because of the electron-donating
Electron-rich is jargon that is used in multiple related meanings with either or both kinetic and thermodynamic implications:
* with regards to electron-transfer, electron-rich species have low ionization energy and/or are reducing agents. Tetr ...
ability of the hydroxyl group (thus weakening the I-OAc bond).
:
Chemoselectivity
Using the standard Dess–Martin periodinane conditions, alcohols can be oxidized to aldehydes/ketones without affectingfuran
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
rings, sulfides
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families of ...
, vinyl ethers, and secondary amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a p ...
. Allylic alcohols are easily oxidized using DMP, which are typically difficult to convert to their respective carbonyls using the typical oxidants.
Myers and coworkers determined that DMP could oxidize N-protected-amino alcohols, without epimerization (unlike most other oxidants, including Swern oxidation). These protected amino alcohols can be very important in the pharmaceutical industry.
Benzylic and allylic alcohols react faster than saturated alcohols, while DMP oxidizes aldoximes and ketoximes to their respective aldehydes and ketones, faster than a primary, secondary or benzylic alcohol to its respective carbonyl.
One example of the Dess–Martin oxidation involves transforming a sensitive α-β-unsaturated alcohol to its corresponding aldehyde. This moiety has been found in several natural products and due to its high functionality, it could be a valuable synthetic building block in organic synthesis. Thongsornkleeb and Danheiser oxidized this sensitive alcohol by employing the Dess Martin Oxidation and altering the work up procedure (diluting with pentanes, washing with poly( 4-vinylpyridine) to remove the acetic acid generated during the reaction, filtering and concentrating via distillation.
:
''t''-Butyl DMP
Difluoro and monofluoro alcohols are more difficult to oxidize.Swern oxidation
In organic chemistry, the Swern oxidation also known as Moffatt-Swern, named after Daniel Swern, is a chemical reaction whereby a primary or secondary Alcohol (chemistry), alcohol () is redox, oxidized to an aldehyde () or ketone () using oxalyl ...
has been used, but a large excess of the oxidant had to be employed, and in some cases did not give reproducible results. Linderman and Graves found DMP was successful in most cases but could not tolerate the presence of nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
functional groups in the alcohol, as these reacted with DMP by displacing acetate. Using the compound shown below produced the desired carbonyls in high yields as the addition of the ''tert''-butoxy group, due to its steric bulk, minimizes these side reactions.
:
See also
*Alcohol oxidation
Alcohol oxidation is a collection of redox, oxidation reactions in organic chemistry that convert Alcohol (chemistry), alcohols to Aldehyde, aldehydes, Ketone, ketones, Carboxylic acid, carboxylic acids, and Ester, esters. The reaction mainly appli ...
* Pyridinium chlorochromate
Pyridinium chlorochromate (PCC) is a yellow-orange salt (chemistry), salt with the chemical formula, formula 5H5NH rO3Cl��. It is a reagent in organic synthesis used primarily for organic redox reaction, oxidation of Alcohol (chemistry), al ...
* Jones oxidation
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of ...
* Oppenauer oxidation
Oppenauer oxidation, named after , is a gentle method for selectively oxidizing secondary alcohols to ketones.
The reaction is the opposite Meerwein–Ponndorf–Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess ...
* Pfitzner–Moffatt oxidation
* Parikh–Doering oxidation
* Albright-Goldman oxidation
* Swern oxidation
In organic chemistry, the Swern oxidation also known as Moffatt-Swern, named after Daniel Swern, is a chemical reaction whereby a primary or secondary Alcohol (chemistry), alcohol () is redox, oxidized to an aldehyde () or ketone () using oxalyl ...
* Corey–Kim oxidation
* Ley oxidation ( TPAP oxidation)
* TEMPO
In musical terminology, tempo (Italian for 'time'; plural 'tempos', or from the Italian plural), measured in beats per minute, is the speed or pace of a given musical composition, composition, and is often also an indication of the composition ...
oxidation
References
External links
Oxidizing agents Periodinanes Acetates Iodine heterocycles Lactones Heterocyclic compounds with 2 rings {{DEFAULTSORT:Dess-Martin periodinane