Decamethylsilicocene on:
[Wikipedia]
[Google]
[Amazon]
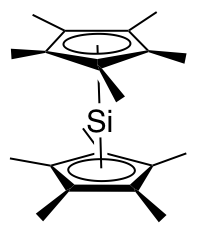 Decamethylsilicocene, (C5Me5)2Si, is a group 14
Decamethylsilicocene, (C5Me5)2Si, is a group 14
 A recent synthesis directly forms decamethylsilicocene through salt metathesis from an
A recent synthesis directly forms decamethylsilicocene through salt metathesis from an 
 Decamethylsilicocene, (C5Me5)2Si, is a group 14
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by hapticity, haptic, covalent bonds to two arene compound, arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ...
. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are ...
s but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.
Synthesis
The first synthesis of decamethylsilicocene was reported by Jutzi and coworkers in 1986. It involved reduction of bis(pentamethylcyclopentadienyl)silicon(IV) dichloride with two equivalents ofsodium naphthalenide
Sodium naphthalene is an organic salt (chemistry), salt with the chemical formula . In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When is ...
to generate decamethylsilicocene, naphthalene, and sodium chloride. Generation of the sterically crowded bis(pentamethylcyclopentadienyl)silicon(IV) dichloride required several steps, beginning with double deprotonation of (C5Me4H)2SiCl2 using ''tert''-butyllithium, followed by treatment of the resultant (C5Me4Li)2SiCl2 with methyl iodide.
Decamethylsilicocene is soluble in aprotic solvent
A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hy ...
s such as hexane
Hexane () or ''n''-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.
Hexane is a colorless liquid, odorless when pure, and with a boiling point of approximately . It is widely used as ...
, benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, and chlorinated solvents. Molecular weight determinations show that decamethylsilicocene exists as a monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
in benzene. The 1H NMR spectrum shows one sharp signal and the 13C- shows two signals one for the ring carbons and one for the methyl group carbons, consistent with the proposed averaged five-fold symmetric structure in solution and η5 coordination of the pentamethylcyclopentadienyl groups.
 A recent synthesis directly forms decamethylsilicocene through salt metathesis from an
A recent synthesis directly forms decamethylsilicocene through salt metathesis from an N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with ...
-stabilized silylene. This synthetic route avoids the synthesis of the bis(pentamethylcyclopentadienyl)silicon(IV) dichloride starting material. In this synthesis, the NHC-stabilized silylene –2,6– was treated with the potassium salt of pentamethylcyclopentadiene at , followed by extraction of decamethylsilicocene into hexane at to remove the NHC and KCl byproducts.

Structure and bonding
The x-ray crystallographically determined structure of decamethylsilicocene contains two isomers in a 2:1 ratio. The major isomer adopts a Cs geometry reminiscent of a bent metallocene, with the cyclopentadienyl planes forming an angle of about 25° and the methyl groups staggered. In this isomer, the lone pair on silicon is described as stereochemically active and the distance from the silicon atom to each Cp* centroid is 2.12 Å. The minor isomer adopts a D5d geometry, the same as decamethylferrocene, with the cyclopentadienyl rings parallel to one another and the methyl groups staggered. The distance from the silicon atom to each Cp* centroid is 2.11 Å. The presence of two isomers is thought to be due to packing effects. Computational studies carried out on the parent silicocene, (C5H5)2Si, reveal a very small (~4 kJ/mol) energetic change upon distorting the molecule from the D5d geometry to either a C2v (bent, hydrogen atoms eclipsed) or Cs (bent, hydrogen atoms staggered) geometry. A qualitative molecular orbital diagram predicts that theHOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
would have silicon(3s)-cyclopentadienyl antibonding character and the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
would have silicon(3p)-cyclopentadienyl antibonding character. NBO calculations are consistent with the predictions from a qualitative molecular orbital diagram, showing antibonding character between the silicon and the cyclopentadienyl ligands in both the HOMO and the LUMO. Calculated NBO valence orbital occupation numbers suggest that significant bonding occurs between the cyclopentadienyl ligands and the silicon 3s, 3px and 3py orbitals.
In comparison, the carbocene congener, silicon is calculated to bond more strongly to the cyclopentadienyl ligands due to the greater radial extension of the 3p orbitals compared to 2p orbitals. Additionally, the energetic separation between the 3s and 3p orbitals is greater than for the 2s and 2p orbitals, leading to less sp mizing which decreases the favorability of distortion to a silylene geometry in which each cyclopentadienyl ligand is bound η1 to the silicon atom. Atoms in molecules (AIM) calculations are consistent with this view. A plot of the Laplacian of the electron density between the central silicon atom and one cyclopentadienyl carbon shows less localization of the charge towards the central atom as compared to equivalent calculations for carbocene.
Reactivity
Decamethylsilicocene reacts with aldehydes and ketones to give products with a silicon (IV) central atom and a carbon-carbon bond formed between two equivalents of the aldehyde or ketone. The two resultant alkoxides are coordinated to the silicon atom to form a five-membered ring. The coordination of the cyclopentadienyl ring changes from η5 to η1 over the course of these reactions Similar changes in the hapticity of the pentamethylcyclopentadientyl rings occur when decamethylsilicocene reacts with carbon-nitrogen triples bonds. With organic cyanates and thiocyanates, carbon-carbon bond formation occurs and the resultant organic fragment is coordinated to the silicon atom through two anionic nitrogens. Decamethylsilicocene reacts with inorganic cyanides such as BrCN and through oxidative addition to form a silicon (IV) product with a cyanide ligand along with either a Br or Me3Si ligand. Decamethylsilicocene can be protonated using strong acids such as . Upon protonation, one equivalent of pentamethylcyclopentadiene is eliminated to produce the pentamethylcyclopentadienylsilicon(II) cation with a . The pentamethylcyclopentadienylsilicon(II) cation reacts with a variety of cyclopentadienyl salts to produce substituted silicocenes. Silicocene derivatives synthesized this way include (Me5C5)((i-Pr)5C5)Si, ((Me5C5)(1,3,4-Me3H2C5)Si and (Me5C5)(H5C5)Si. The latter compound is stable at but begins to decompose at . Additionally, the pentamethylcyclopentadienylsilicon(II) cation can react with metal precursors to generate complexes with metal-silicon multiple bonds.References
{{reflist Sandwich compounds Silicon compounds