cyclometalation on:
[Wikipedia]
[Google]
[Amazon]
In
 Many compounds containing metals in rings are known, for example
Many compounds containing metals in rings are known, for example

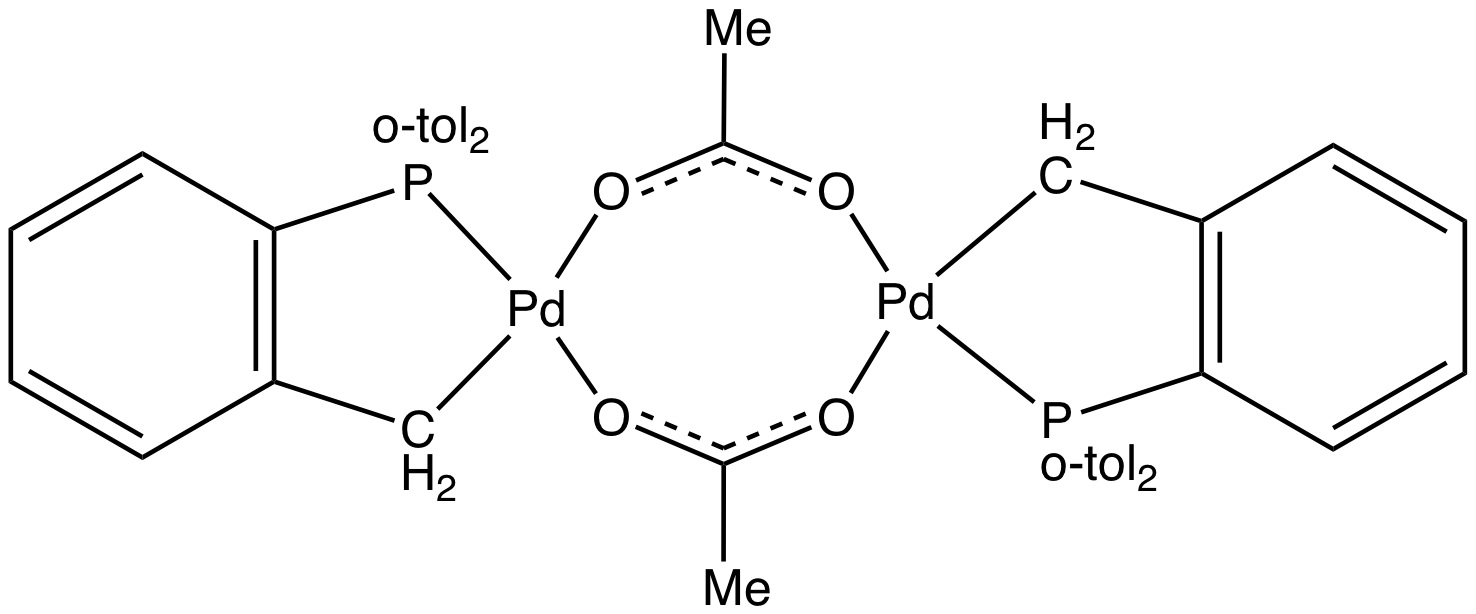 Metallacycles often arise by cyclization of arene-containing donor ligands, e.g. aryl phosphines and amines. An early example is the cyclization of IrCl(PPh3)3 to give the corresponding Ir(III) hydride containing a four-membered IrPCC ring. Palladium(II) and platinum(II) have long been known to ortho-metalate aromatic ligands such as
Metallacycles often arise by cyclization of arene-containing donor ligands, e.g. aryl phosphines and amines. An early example is the cyclization of IrCl(PPh3)3 to give the corresponding Ir(III) hydride containing a four-membered IrPCC ring. Palladium(II) and platinum(II) have long been known to ortho-metalate aromatic ligands such as
organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
center; this is to some extent similar to heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
s. Metallacycles appear frequently as reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s in catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, e.g. olefin metathesis
In organic chemistry, Olefin Metathesis or Alkene Metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the Bond cleavage, scission and regeneration of carbon-carbon double bonds. Because of the ...
and alkyne trimerization. In organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, directed ortho metalation
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium co ...
is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.
Nomenclature
Typically, metallacycles are cyclic compounds with two metal carbon bonds. Many compounds containing metals in rings are known, for example
Many compounds containing metals in rings are known, for example chelate
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
rings. Usually, such compounds are not classified as metallacycles, but the naming conventions are not rigidly followed. Within the area of coordination chemistry
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
and supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
, examples include metallacrowns, metallacryptands, metallahelices, and molecular wheels.
Classes of metallacycles
Metal-alkene complexes can be viewed as the smallest metallacycles, but they usually are not classified as metallacycles. In theDewar–Chatt–Duncanson model
The Dewar–Chatt–Duncanson model is a model in organometallic chemistry that explains the chemical bonding in transition metal alkene complexes. The model is named after Michael J. S. Dewar, Joseph Chatt and L. A. Duncanson.
The alkene don ...
, one resonance structure for the M(η2-alkene) center is the metallacyclopropane.

Metallacyclobutanes
The parent metallacyclobutane has the formula LnM(CH2)3 where L is a ligand attached to M. A stable example is ( PPh3)2Pt(CH2)3. The first example was prepared by oxidative addition of cyclopropane to platinum. Metallacyclobutane intermediates are involved in the alkene metathesis and in the oligomerization and dimerization of ethylene. In alkene metathesis, the Chauvin mechanism invokes the attack of an alkene at an electrophilic metal carbene catalyst. This work helped to validate the Chauvin mechanism for olefin metathesis.Metallacyclopentadienes and metallabenzenes
The parent metallacyclopentadiene, or metallole, has the formula LnM(CH)4. Most arise from the coupling of twoalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s at a low valent metal centers such as derivatives of Co(I) and Zr(II). Late metal derivatives (Co, Ni) are intermediates in the metal-catalysed trimerization of alkynes to arenes. Early metal derivatives, i.e. derivatives of Ti and Zr, are used stoichiometrically. For example, the zirconacyclopentadiene Cp2ZrC4Me4 is a useful carrier for C4Me42−. Some of the oldest metallacycles are the ferroles, which are dimetallacyclopentadiene complexes of the formula Fe2(C2R4)(CO)6. They are derived from coupling of alkynes as well as from the desulfurization of thiophenes.
The parent metallacyclobenzene
The metallabenzenes are class of chemical compound of the form LnM(CH)5, or derivatives thereof. Most metallabenzenes do not feature the M(CH)5 ring itself, but, instead, some of the H atoms are replaced by other substituents. The parent metallabe ...
s have the formula LnM(CH)5. They can be viewed as derivatives of benzene wherein a CH center has been replaced by a transition metal complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
.
Metallacyclopentanes
The parent metallacyclopentane has the formula LnM(CH2)4. Such compounds are intermediates in the metal catalysed dimerization, trimerization, and tetramerization of ethylene to give but-1-ene, hex-1-ene and oct-1-ene, respectively. Metallacyclopentanes are invoked as intermediates in the evolution of heterogeneous alkene metathesis catalysts from ethylene and metal oxides. Metallacyclopentane intermediates are proposed to isomerize to metallacyclobutanes, which then eliminate propylene giving the alkylidene.Ortho-metalation
 Metallacycles often arise by cyclization of arene-containing donor ligands, e.g. aryl phosphines and amines. An early example is the cyclization of IrCl(PPh3)3 to give the corresponding Ir(III) hydride containing a four-membered IrPCC ring. Palladium(II) and platinum(II) have long been known to ortho-metalate aromatic ligands such as
Metallacycles often arise by cyclization of arene-containing donor ligands, e.g. aryl phosphines and amines. An early example is the cyclization of IrCl(PPh3)3 to give the corresponding Ir(III) hydride containing a four-membered IrPCC ring. Palladium(II) and platinum(II) have long been known to ortho-metalate aromatic ligands such as azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide c ...
, benzylamines, and 2-phenylpyridines. These reactions are strongly influenced by substituent effects, including the Thorpe-Ingold effect. Ligands that lack aryl substituents will sometimes cyclometalate via activation of methyl groups, an early example being the internal oxidative addition of methylphosphine ligands. Metallacycle formation interferes with intermolecular C-H activation processes. For this reason, specialized " pincer ligands" ligands have been developed that resist ortho-metalation.
References
{{Reflist Organometallic chemistry Heterocyclic compounds