cumulene on:
[Wikipedia]
[Google]
[Amazon]
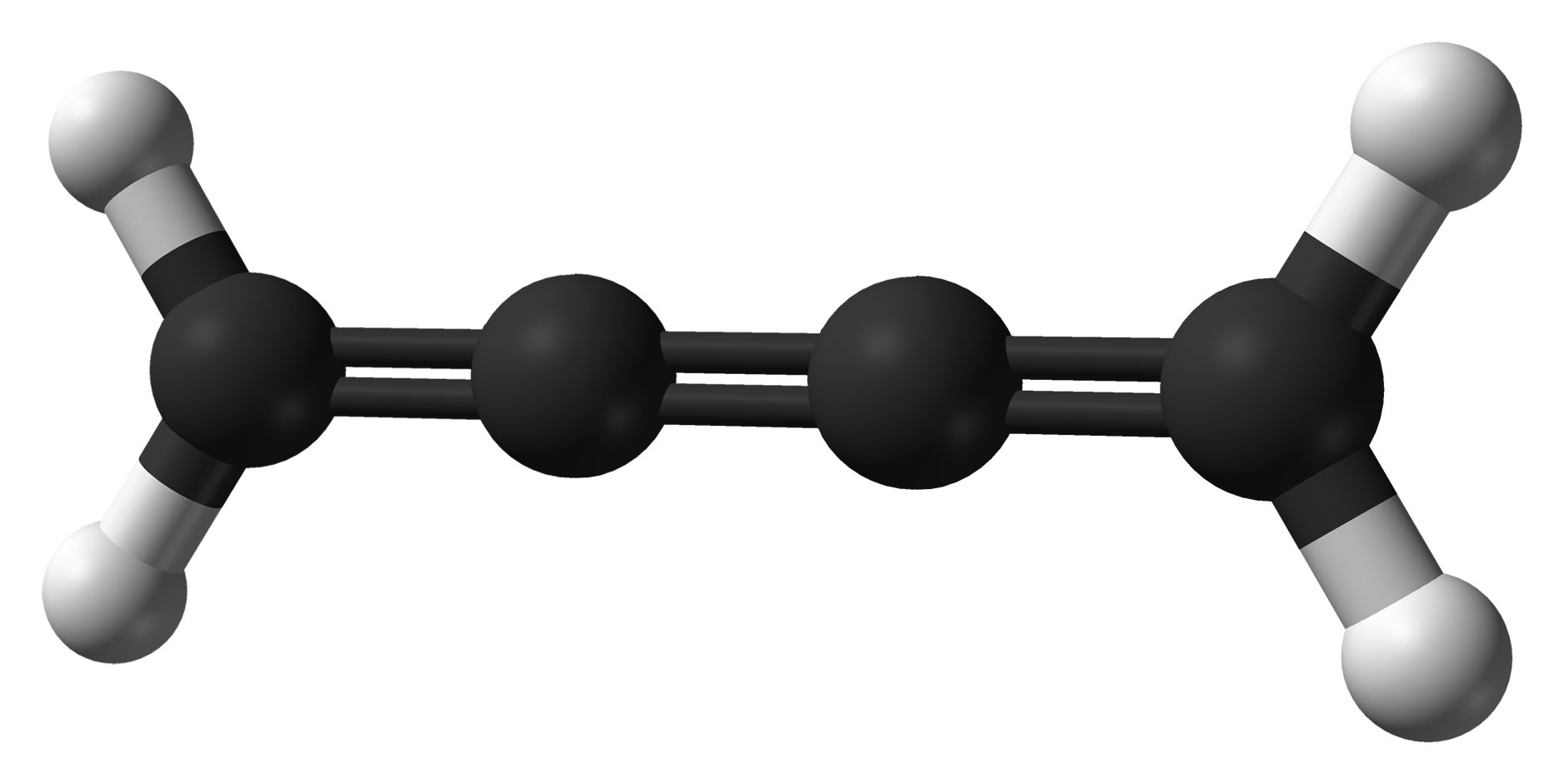 A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to
 The rigidity of cumulenes arises from the fact that the internal carbon atoms carry double bonds. Their sp hybridisation results in two π bonds, one to each neighbor, which are perpendicular to each other. This bonding reinforces a linear geometry of the carbon chain.
Cumulenes with non-equivalent substituents on each end exhibit isomerism. If the number of consecutive double bonds is odd, there is ''cis''–''trans'' isomerism as for alkenes. If the number of consecutive double bonds is even, there is axial chirality as for allenes.
The rigidity of cumulenes arises from the fact that the internal carbon atoms carry double bonds. Their sp hybridisation results in two π bonds, one to each neighbor, which are perpendicular to each other. This bonding reinforces a linear geometry of the carbon chain.
Cumulenes with non-equivalent substituents on each end exhibit isomerism. If the number of consecutive double bonds is odd, there is ''cis''–''trans'' isomerism as for alkenes. If the number of consecutive double bonds is even, there is axial chirality as for allenes.
 A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
s, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...
s and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide.
Synthesis
The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalo vinylidene. Tetraphenylbutatriene was reported synthesized in 1977 by homocoupling of 2,2-diphenyl-1,1,1-tribromoethane with elemental copper in dimethylformamide.Structure
 The rigidity of cumulenes arises from the fact that the internal carbon atoms carry double bonds. Their sp hybridisation results in two π bonds, one to each neighbor, which are perpendicular to each other. This bonding reinforces a linear geometry of the carbon chain.
Cumulenes with non-equivalent substituents on each end exhibit isomerism. If the number of consecutive double bonds is odd, there is ''cis''–''trans'' isomerism as for alkenes. If the number of consecutive double bonds is even, there is axial chirality as for allenes.
The rigidity of cumulenes arises from the fact that the internal carbon atoms carry double bonds. Their sp hybridisation results in two π bonds, one to each neighbor, which are perpendicular to each other. This bonding reinforces a linear geometry of the carbon chain.
Cumulenes with non-equivalent substituents on each end exhibit isomerism. If the number of consecutive double bonds is odd, there is ''cis''–''trans'' isomerism as for alkenes. If the number of consecutive double bonds is even, there is axial chirality as for allenes.
Transition metal heterocumulenes
The first reported complex containing a vinylidene ligand was (Ph2C2Fe2(CO)8, derived from the reaction of diphenylketene and Fe(CO)5. Structurally, this molecule resembles Fe2(CO)9, wherein one ''μ''-CO ligand is replaced by 1,1-diphenylvinylidene, Ph2C2. The first monometallic vinylidene complex was ( C5H5)Mo( P(C6H5)3)(CO)2 =C(CN)2l.See also
* Cyclopropatriene and cyclohexahexaene, cyclic cumulenesReferences
{{Authority control Astrochemistry