Control Rods on:
[Wikipedia]
[Google]
[Amazon]
 Control rods are used in
Control rods are used in
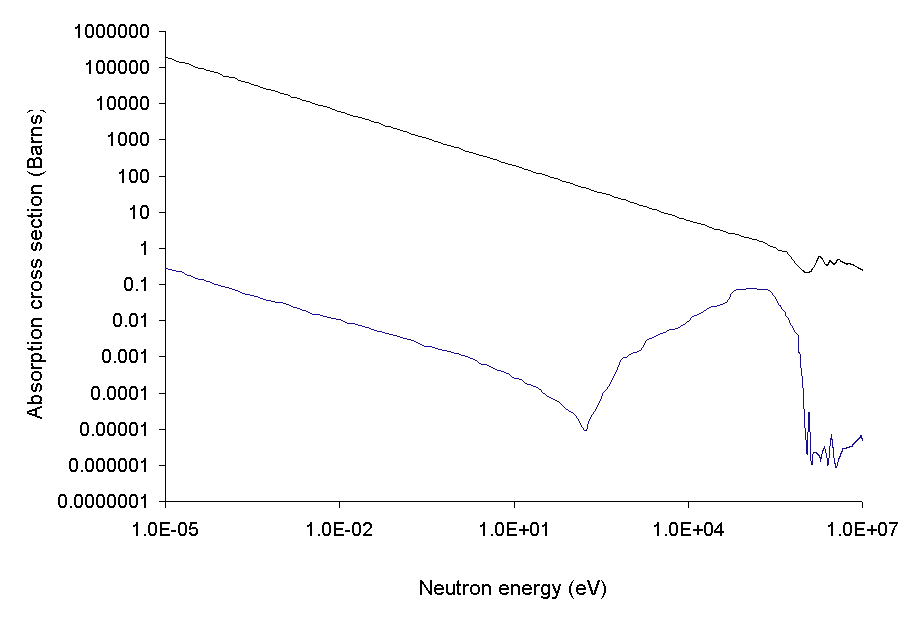 Chemical elements with usefully high neutron capture cross-sections include
Chemical elements with usefully high neutron capture cross-sections include
 Control rods are used in
Control rods are used in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s to control the rate of fission of the nuclear fuel – uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
or plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
. Their compositions include chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s such as boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
, cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
, silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
, hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, or indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
, that are capable of absorbing many neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s without themselves decaying. These elements have different neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
cross sections for neutrons of various energies. Boiling water reactor
A boiling water reactor (BWR) is a type of nuclear reactor used for the generation of electrical power. It is the second most common type of electricity-generating nuclear reactor after the pressurized water reactor (PWR).
BWR are thermal neutro ...
s (BWR), pressurized water reactor
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan, India and Canada).
In a PWR, water is used both as ...
s (PWR), and heavy-water reactor
A heavy water reactor (HWR) is a type of nuclear reactor which uses heavy water (D2O, deuterium oxide) as a neutron moderator. It may also use this as the coolant, in the case of Pressurized heavy water reactor, pressurized heavy water reactors. D ...
s (HWR) operate with thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium wit ...
s, while breeder reactor
A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. These reactors can be fueled with more-commonly available isotopes of uranium and thorium, such as uranium-238 and thorium-232, as opposed to the ...
s operate with fast neutrons. Each reactor design can use different control rod materials based on the energy spectrum of its neutrons. Control rods have been used in nuclear aircraft engines like Project Pluto as a method of control.
Operating principle
Control rods are inserted into the core of a nuclear reactor and adjusted in order to control the rate of thenuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series or "positive feedback loop" of thes ...
and, thereby, the thermal power output of the reactor, the rate of steam
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is inv ...
production, and the electrical power
Electric power is the rate of transfer of electrical energy within a electric circuit, circuit. Its SI unit is the watt, the general unit of power (physics), power, defined as one joule per second. Standard prefixes apply to watts as with oth ...
output of the power station.
The number of control rods inserted, and the distance to which they are inserted, strongly influence the ''reactivity'' of the reactor. When reactivity (as effective neutron multiplication factor) is above 1, the rate of the nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series or "positive feedback loop" of thes ...
increases exponentially over time. When reactivity is below 1, the rate of the reaction decreases exponentially over time. When all control rods are fully inserted, they keep reactivity barely above 0, which quickly slows a running reactor to a stop and keeps it stopped (in shutdown). If all control rods are fully removed, reactivity is significantly above 1, and the reactor quickly runs hotter and hotter, until some other factor (such as temperature reactivity feedback) slows the reaction rate. Maintaining a constant power output requires keeping the long-term average neutron multiplication factor close to 1.
A new reactor is assembled with its control rods fully inserted. Control rods are partially removed from the core to allow the nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series or "positive feedback loop" of thes ...
to start up and increase to the desired power level. Neutron flux
The neutron flux is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total distance travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling ...
can be measured, and is roughly proportional to reaction rate and power level. To increase power output, some control rods are pulled out a small distance for a while. To decrease power output, some control rods are pushed in a small distance for a while. Several other factors affect the reactivity; to compensate for them, an automatic control system adjusts the control rods small amounts in or out, as-needed in some reactors. Each control rod influences some part of the reactor more than others; calculated adjustments to fuel distribution can be made to maintain similar reaction rates and temperatures in different parts of the core.
Typical shutdown time for modern reactors such as the European Pressurized Reactor or Advanced CANDU reactor is two seconds for 90% reduction, limited by decay heat
Decay heat is the heat released as a result of radioactive decay. This heat is produced as an effect of radiation on materials: the energy of the alpha particle, alpha, Beta particle, beta or gamma radiation is converted into the thermal movement ...
.
Control rods are usually used in control rod assemblies (typically 20 rods for a commercial PWR assembly) and inserted into guide tubes within the fuel elements. Control rods often stand vertically within the core. In PWRs they are inserted from above, with the control rod drive mechanisms mounted on the reactor pressure vessel
A pressure vessel is a container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.
Construction methods and materials may be chosen to suit the pressure application, and will depend on the size o ...
head. In BWRs, due to the necessity of a steam dryer above the core, this design requires insertion of the control rods from beneath.
Materials
 Chemical elements with usefully high neutron capture cross-sections include
Chemical elements with usefully high neutron capture cross-sections include silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
, indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
, and cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
. Other candidate elements include boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
, cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
, hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, samarium, europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
, gadolinium
Gadolinium is a chemical element; it has Symbol (chemistry), symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moi ...
, terbium
Terbium is a chemical element; it has Symbol (chemistry), symbol Tb and atomic number 65. It is a silvery-white, rare earth element, rare earth metal that is malleable and ductile. The ninth member of the lanthanide series, terbium is a fairly ele ...
, dysprosium
Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it ...
, holmium
Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other ...
, erbium
Erbium is a chemical element; it has Symbol (chemistry), symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare- ...
, thulium
Thulium is a chemical element; it has symbol Tm and atomic number 69. It is the thirteenth element in the lanthanide series of metals. It is the second-least abundant lanthanide in the Earth's crust, after radioactively unstable promethium. It i ...
, ytterbium, and lutetium
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
. Alloys or compounds may also be used, such as high-boron steel
Boron steel refers to steel alloyed with a small amount of boron, usually less than 1%. The addition of boron to steel greatly increases the hardenability of the resulting alloy.
Description
Boron is added to steel as ferroboron (~12-24% B). As t ...
, silver-indium-cadmium alloy, boron carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders,
as well as numerous industrial applications. With a Vickers har ...
, zirconium diboride, titanium diboride, hafnium diboride, gadolinium nitrate, gadolinium titanate, dysprosium titanate, and boron carbide–europium hexaboride composite.
The material choice is influenced by the neutron energy in the reactor, their resistance to neutron-induced swelling
Neutron-induced swelling is the increase of volume and decrease of density of materials subjected to intense neutron radiation. Neutrons impacting the material's lattice rearrange its atoms, causing buildup of dislocations, voids, and Wigner ene ...
, and the required mechanical and lifespan properties. The rods may have the form of tubes filled with neutron-absorbing pellets or powder. The tubes can be made of stainless steel or other "neutron window" materials such as zirconium, chromium, silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder a ...
, or cubic (cubic boron nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexago ...
).
The burnup of " burnable poison" isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s also limits lifespan of a control rod. They may be reduced by using an element such as hafnium, a "non-burnable poison" which captures multiple neutrons before losing effectiveness, or by not using neutron absorbers for trimming. For example, in pebble bed reactor
The pebble-bed reactor (PBR) is a design for a graphite- moderated, gas-cooled nuclear reactor. It is a type of very-high-temperature reactor (VHTR), one of the six classes of nuclear reactors in the Generation IV initiative.
The basic desig ...
s or in possible new type lithium-7-moderated and -cooled reactors that use fuel and absorber pebbles.
Some rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set o ...
s are excellent neutron absorbers and are more common than silver (reserves of about 500,000t). For example, ytterbium (reserves about one M tons) and yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
, 400 times more common, with middle capturing values, can be found and used together without separation inside minerals like xenotime (Yb) (Yb0.40Y0.27Lu0.12Er0.12Dy0.05Tm0.04Ho0.01)PO4, or keiviite (Yb) (Yb1.43Lu0.23Er0.17Tm0.08Y0.05Dy0.03Ho0.02)2Si2O7, lowering the cost. Xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
is also a strong neutron absorber as a gas, and can be used for controlling and (emergency) stopping helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
-cooled reactors, but does not function in cases of pressure loss, or as a burning protection gas together with argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
around the vessel part especially in case of core catching reactors or if filled with sodium or lithium. Fission-produced xenon can be used after waiting for caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
to precipitate, when practically no radioactivity is left. Cobalt-59 is also used as an absorber for winning of cobalt-60 for use as a gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
source. Control rods can also be constructed as thick turnable rods with a tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
reflector and absorber side turned to stop by a spring in less than one second.
Silver-indium-cadmium alloys, generally 80% Ag, 15% In, and 5% Cd, are a common control rod material for pressurized water reactor
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan, India and Canada).
In a PWR, water is used both as ...
s. The somewhat different energy absorption regions of the materials make the alloy an excellent neutron absorber
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable ef ...
. It has good mechanical strength and can be easily fabricated. It must be encased in stainless steel to prevent corrosion in hot water. Although indium is less rare than silver, it is more expensive.
Boron is another common neutron absorber. Due to the different cross sections of 10B and 11B, materials containing boron enriched in 10B by isotopic separation are frequently used. The wide absorption spectrum of boron also makes it suitable as a neutron shield. The mechanical properties of boron in its elementary form are unsuitable, and therefore alloys or compounds have to be used instead. Common choices are high-boron steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
and boron carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders,
as well as numerous industrial applications. With a Vickers har ...
. The latter is used as a control rod material in both PWRs and BWRs. 10B/11B separation is done commercially with gas centrifuge
A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centrifugal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radiu ...
s over BF3, but can also be done over BH3 from borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a c ...
production or directly with an energy optimized melting centrifuge, using the heat of freshly separated boron for preheating.
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
has excellent properties for reactors using water for both moderation and cooling. It has good mechanical strength, can be easily fabricated, and is resistant to corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
in hot water. Hafnium can be alloyed with other elements, e.g. with tin and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
to increase tensile and creep strength, with iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
, and niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
for corrosion resistance, and with molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
for wear resistance, hardness, and machineability. Such alloys are designated as Hafaloy, Hafaloy-M, Hafaloy-N, and Hafaloy-NM. The high cost and low availability of hafnium limit its use in civilian reactors, although it is used in some US Navy
The United States Navy (USN) is the naval warfare, maritime military branch, service branch of the United States Department of Defense. It is the world's most powerful navy with the largest Displacement (ship), displacement, at 4.5 millio ...
reactors. Hafnium carbide can also be used as an insoluble material with a high melting point of 3890 °C and density higher than that of uranium dioxide for sinking, unmelted, through corium.
Dysprosium titanate was undergoing evaluation for pressurized water control rods. Dysprosium titanate is a promising replacement for Ag-In-Cd alloys because it has a much higher melting point, does not tend to react with cladding materials, is easy to produce, does not produce radioactive waste, does not swell and does not outgas. It was developed in Russia and is recommended by some for VVER
The water-water energetic reactor (WWER), or VVER (from ) is a series of pressurized water reactor designs originally developed in the Soviet Union, and now Russia, by OKB Gidropress. The idea of such a reactor was proposed at the Kurchatov Instit ...
and RBMK reactors. A disadvantage is less titanium and oxide absorption, that other neutron absorbing elements do not react with the already high-melting point cladding materials and that just using the unseparated content with dysprosium inside of minerals like Keiviit Yb inside chromium, SiC or c11B15N tubes deliver superior price and absorption without swelling and outgassing.
Hafnium diboride is another such material. It can be used alone or in a sintered mixture of hafnium and boron carbide powders.
Many other compounds of rare-earth elements can be used, such as samarium with boron-like europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
and samarium boride, which is already used in the colour industry. Less absorptive compounds of boron similar to titanium, but inexpensive, such as molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
as Mo2B5. Since they all swell with boron, in practice other compounds are better, such as carbides, or compounds with two or more neutron-absorbing elements together. It is important that tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, and probably also other elements such as tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
, have much the same high capture qualities as hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, but with the opposite effect. This is not explainable by neutron reflection alone. An obvious explanation is resonance gamma rays increasing the fission and breeding ratio versus causing greater capture of uranium, and others over metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
conditions such as for isotope 235mU, which has a half-life of approximately 26 minutes.
Additional means of reactivity regulation
Other means of controlling reactivity include (for PWR) a soluble neutron absorber (boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white ...
) added to the reactor coolant, allowing the complete extraction of the control rods during stationary power operation, ensuring an even power and flux distribution over the entire core. This chemical shim, along with the use of burnable neutron poisons within the fuel pellets, is used to assist regulation of the core's long term reactivity, while the control rods are used for rapid reactor power changes (e.g. shutdown and start up). Operators of BWRs use the coolant flow through the core to control reactivity by varying the speed of the reactor recirculation pumps (an increase in coolant flow through the core improves the removal of steam bubbles, thus increasing the density of the coolant/ moderator, increasing power).
Safety
In most reactor designs, as a safety measure, control rods are attached to the lifting machinery byelectromagnet
An electromagnet is a type of magnet in which the magnetic field is produced by an electric current. Electromagnets usually consist of wire (likely copper) wound into a electromagnetic coil, coil. A current through the wire creates a magnetic ...
s, rather than direct mechanical linkage. This means that in the event of power failure, or if manually invoked due to failure of the lifting machinery, the control rods fall automatically, under gravity, all the way into the pile to stop the reaction. A notable exception to this fail-safe
In engineering, a fail-safe is a design feature or practice that, in the event of a failure causes, failure of the design feature, inherently responds in a way that will cause minimal or no harm to other equipment, to the environment or to people. ...
mode of operation is the BWR, which requires hydraulic insertion in the event of an emergency shut-down, using water from a special tank under high pressure. Quickly shutting down a reactor in this way is called scram
A scram or SCRAM is an emergency shutdown of a nuclear reactor effected by immediately terminating the fission reaction. It is also the name that is given to the manually operated kill switch that initiates the shutdown. In commercial reactor ...
ming.
Criticality accident prevention
Mismanagement or control rod failure have often been blamed fornuclear accident
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility." Examples include radiation poisoning, lethal effect ...
s, including the SL-1
Stationary Low-Power Reactor Number One, also known as SL-1, initially the Argonne Low Power Reactor (ALPR), was a United States Army experimental nuclear reactor in the Western United States, western United States at the Idaho National Laborato ...
explosion and the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
.
''Homogeneous'' neutron absorbers have often been used to manage criticality accident
A criticality accident is an accidental uncontrolled nuclear fission chain reaction. It is sometimes referred to as a critical excursion, critical power excursion, divergent chain reaction, or simply critical. Any such event involves the uninten ...
s which involve aqueous solutions of fissile
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal Nuclear chain reaction#Fission chain reaction, chain reaction can only be achieved with fissil ...
metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
s. In several such accidents, either borax
The BORAX Experiments were a series of safety experiments on boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
(sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
) or a cadmium compound has been added to the system. The cadmium can be added as a metal to nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
solutions of fissile material; the corrosion of the cadmium in the acid will then generate cadmium nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
''in situ''.
In carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
-cooled reactors such as the AGR, if the solid control rods fail to arrest the nuclear reaction, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
gas can be injected into the primary coolant cycle. This is because nitrogen has a larger absorption cross-section for neutrons than carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
or oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
; hence, the core then becomes less reactive.
As the neutron energy increases, the neutron cross section of most isotopes decreases. The boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
10B is responsible for the majority of the neutron absorption. Boron-containing materials can also be used as neutron shielding, to reduce the activation
In chemistry and biology, activation is the process whereby something is prepared or excited for a subsequent reaction.
Chemistry
In chemistry, "activation" refers to the reversible transition of a molecule into a nearly identical chemical or ...
of material close to a reactor core.
See also
*Nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by ...
*Nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
*Nuclear safety
Nuclear safety is defined by the International Atomic Energy Agency (IAEA) as "The achievement of proper operating conditions, prevention of accidents or mitigation of accident consequences, resulting in protection of workers, the public and the ...
* Wigner effect
Notes
References
External links
Further reading
* * * {{Authority control Alloys Nuclear power plant components Nuclear reactor safety Pressurized water reactors