click reaction on:
[Wikipedia]
[Google]
[Amazon]
Click chemistry is an approach to chemical synthesis that emphasizes efficiency, simplicity, selectivity, and modularity in chemical processes used to join molecular building blocks. It includes both the development and use of "click reactions", a set of simple, biocompatible chemical reactions that meet specific criteria like high yield, fast reaction rates, and minimal byproducts. It was first fully described by K. Barry Sharpless, Hartmuth C. Kolb, and M. G. Finn of
 The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery V. Fokin and K. Barry Sharpless at the Scripps Research Institute in
The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery V. Fokin and K. Barry Sharpless at the Scripps Research Institute in 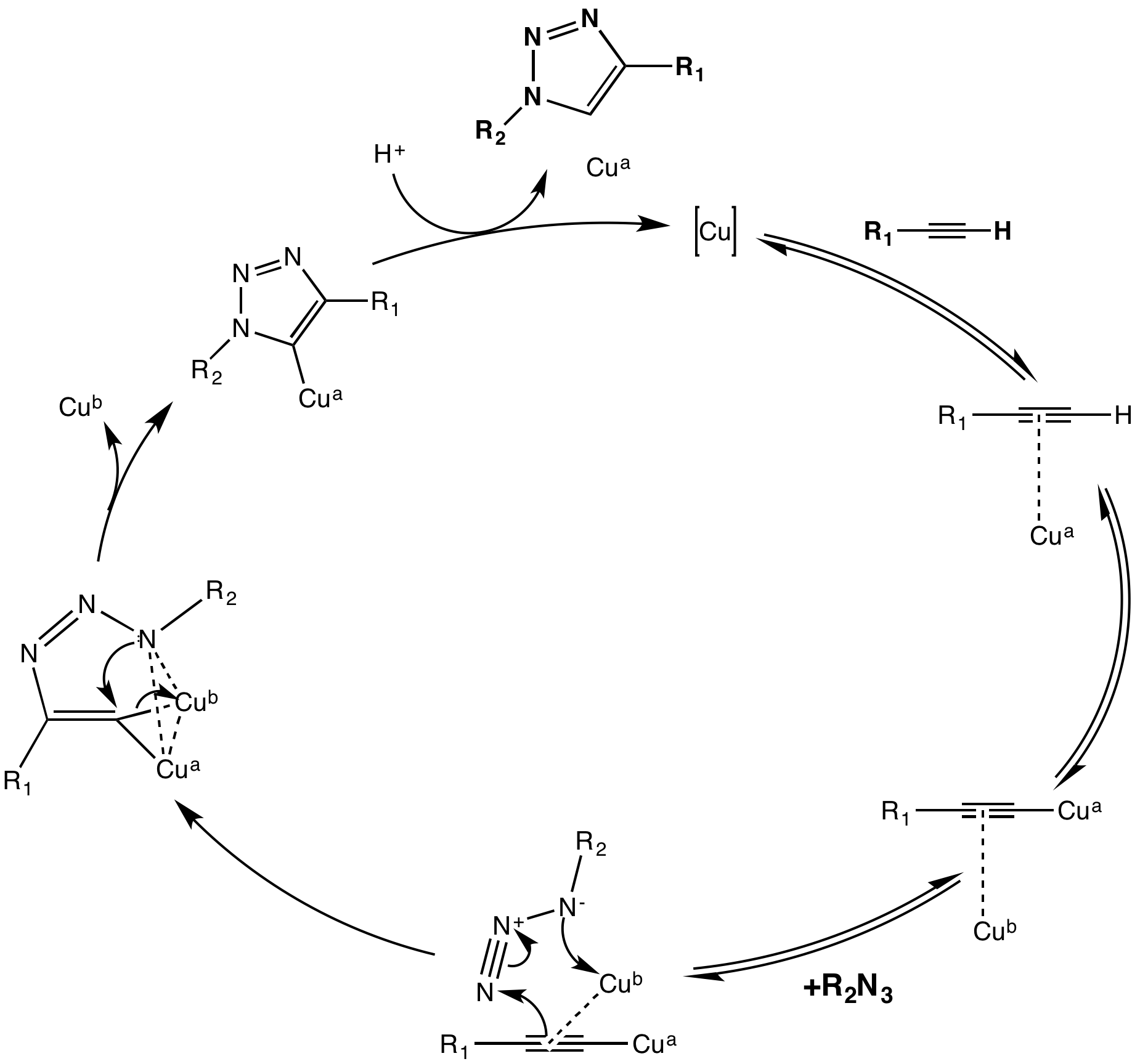 Moreover, this copper-catalyzed "click" does not require ligands on the metal, although accelerating ligands such as tris(triazolyl)methyl amine ligands with various substituents have been reported and used with success in aqueous solution. Other ligands such as PPh3 and TBIA can also be used, even though PPh3 is liable to Staudinger ligation with the azide substituent. Cu2O in water at room temperature was found also to catalyze the same reaction in 15 minutes with 91% yield.
The first reaction mechanism proposed included one catalytic copper atom; but isotope, kinetic, and other studies have suggested a dicopper mechanism may be more relevant. Even though this reaction proceeds effectively at biological conditions, copper in this range of dosage is cytotoxic. Solutions to this problem have been presented, such as using water-soluble ligands on the copper to enhance cell penetration of the catalyst and thereby reduce the dosage needed,Brotherton, W. S.; Michaels, H. A.; Simmons, J. T.; Clark, R.J.; Dalal, N. S.; Zhu, L. Org. Lett. 2009, 11, 4954. or to use chelating ligands to further increase the effective concentration of Cu(I) and thereby decreasing the actual dosage.Alder, K.; Stein, G.; Finzenhagen, H. Justus Liebigs Ann.Chem 1931, 485, 211.
Although the Cu(I)-catalyzed variant was first reported by Meldal and co-workers for the synthesis of peptidotriazoles on solid support, their conditions were far from the true spirit of click chemistry and were overtaken by the publicly more recognized Sharpless. Meldal and co-workers also chose not to label this reaction type "click chemistry" which allegedly caused their discovery to be largely overlooked by the mainstream chemical society. Fokin and Sharpless independently described it as a reliable catalytic process offering "an unprecedented level of selectivity, reliability, and scope for those organic synthesis endeavors which depend on the creation of covalent links between diverse building blocks".
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.
Moreover, this copper-catalyzed "click" does not require ligands on the metal, although accelerating ligands such as tris(triazolyl)methyl amine ligands with various substituents have been reported and used with success in aqueous solution. Other ligands such as PPh3 and TBIA can also be used, even though PPh3 is liable to Staudinger ligation with the azide substituent. Cu2O in water at room temperature was found also to catalyze the same reaction in 15 minutes with 91% yield.
The first reaction mechanism proposed included one catalytic copper atom; but isotope, kinetic, and other studies have suggested a dicopper mechanism may be more relevant. Even though this reaction proceeds effectively at biological conditions, copper in this range of dosage is cytotoxic. Solutions to this problem have been presented, such as using water-soluble ligands on the copper to enhance cell penetration of the catalyst and thereby reduce the dosage needed,Brotherton, W. S.; Michaels, H. A.; Simmons, J. T.; Clark, R.J.; Dalal, N. S.; Zhu, L. Org. Lett. 2009, 11, 4954. or to use chelating ligands to further increase the effective concentration of Cu(I) and thereby decreasing the actual dosage.Alder, K.; Stein, G.; Finzenhagen, H. Justus Liebigs Ann.Chem 1931, 485, 211.
Although the Cu(I)-catalyzed variant was first reported by Meldal and co-workers for the synthesis of peptidotriazoles on solid support, their conditions were far from the true spirit of click chemistry and were overtaken by the publicly more recognized Sharpless. Meldal and co-workers also chose not to label this reaction type "click chemistry" which allegedly caused their discovery to be largely overlooked by the mainstream chemical society. Fokin and Sharpless independently described it as a reliable catalytic process offering "an unprecedented level of selectivity, reliability, and scope for those organic synthesis endeavors which depend on the creation of covalent links between diverse building blocks".
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.
 This reaction proceeds as a concerted +2cycloaddition to the triple bond in a cyclooctyne in the same mechanism as the Huisgen 1,3-dipolar cycloaddition. Substituents other than fluorines, such as benzene rings, are also allowed on the cyclooctyne.
This reaction has been used successfully to probe for azides in living systems, even though the reaction rate is somewhat slower than that of the CuAAC. Moreover, because the synthesis of cyclooctynes often gives low yield, probe development for this reaction has not been as rapid as for other reactions. But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO or DBCO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.
This reaction proceeds as a concerted +2cycloaddition to the triple bond in a cyclooctyne in the same mechanism as the Huisgen 1,3-dipolar cycloaddition. Substituents other than fluorines, such as benzene rings, are also allowed on the cyclooctyne.
This reaction has been used successfully to probe for azides in living systems, even though the reaction rate is somewhat slower than that of the CuAAC. Moreover, because the synthesis of cyclooctynes often gives low yield, probe development for this reaction has not been as rapid as for other reactions. But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO or DBCO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.
 Because this reaction is metal-free and proceeds with fast kinetics (k2 as fast as 60 1/Ms, faster than both the CuAAC or the SPAAC) SPANC can be used for live cell labeling. Moreover, substitution on both the carbon and nitrogen atoms of the nitrone dipole, and acyclic and endocyclic nitrones are all tolerated. This large allowance provides a lot of flexibility for nitrone handle or probe incorporation.
However, the isoxazoline product is not as stable as the triazole product of the CuAAC and the SpAAC, and can undergo rearrangements at biological conditions. Regardless, this reaction is still very useful as it has notably fast reaction kinetics.
The applications of this reaction include labeling proteins containing serine as the first residue: the serine is oxidized to aldehyde with NaIO4 and then converted to nitrone with p-methoxybenzenethiol, N-methylhydroxylamine and p-ansidine, and finally incubated with cyclooctyne to give a click product. The SPANC also allows for multiplex labeling.
Because this reaction is metal-free and proceeds with fast kinetics (k2 as fast as 60 1/Ms, faster than both the CuAAC or the SPAAC) SPANC can be used for live cell labeling. Moreover, substitution on both the carbon and nitrogen atoms of the nitrone dipole, and acyclic and endocyclic nitrones are all tolerated. This large allowance provides a lot of flexibility for nitrone handle or probe incorporation.
However, the isoxazoline product is not as stable as the triazole product of the CuAAC and the SpAAC, and can undergo rearrangements at biological conditions. Regardless, this reaction is still very useful as it has notably fast reaction kinetics.
The applications of this reaction include labeling proteins containing serine as the first residue: the serine is oxidized to aldehyde with NaIO4 and then converted to nitrone with p-methoxybenzenethiol, N-methylhydroxylamine and p-ansidine, and finally incubated with cyclooctyne to give a click product. The SPANC also allows for multiplex labeling.
 Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro +2 cycloaddition (see figure). Like the other reactions of the trans-cyclooctene, ring strain release is a driving force for this reaction. Thus, three-membered and four-membered cycloalkenes, due to their high ring strain, make ideal alkene substrates.
Similar to other +2cycloadditions, electron-donating substituents on the dienophile and electron-withdrawing substituents on the diene accelerate the inverse-demand Diels-Alder. The diene, the tetrazine, by virtue of having the additional nitrogens, is a good diene for this reaction. The dienophile, the activated alkene, can often be attached to electron-donating alkyl groups on target molecules, thus making the dienophile more suitable for the reaction.
Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro +2 cycloaddition (see figure). Like the other reactions of the trans-cyclooctene, ring strain release is a driving force for this reaction. Thus, three-membered and four-membered cycloalkenes, due to their high ring strain, make ideal alkene substrates.
Similar to other +2cycloadditions, electron-donating substituents on the dienophile and electron-withdrawing substituents on the diene accelerate the inverse-demand Diels-Alder. The diene, the tetrazine, by virtue of having the additional nitrogens, is a good diene for this reaction. The dienophile, the activated alkene, can often be attached to electron-donating alkyl groups on target molecules, thus making the dienophile more suitable for the reaction.
 Methods for the incorporation of click reaction partners into systems in and ex vivo contribute to the scope of possible reactions. The development of unnatural amino acid incorporation by ribosomes has allowed for the incorporation of click reaction partners as unnatural side groups on these unnatural amino acids. For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid. In another example, "CpK" has a side group including a cyclopropane alpha to an amide bond that serves as a reaction partner to tetrazine in an inverse diels-alder reaction.
Methods for the incorporation of click reaction partners into systems in and ex vivo contribute to the scope of possible reactions. The development of unnatural amino acid incorporation by ribosomes has allowed for the incorporation of click reaction partners as unnatural side groups on these unnatural amino acids. For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid. In another example, "CpK" has a side group including a cyclopropane alpha to an amide bond that serves as a reaction partner to tetrazine in an inverse diels-alder reaction.
 The synthesis of
The synthesis of
Click Chemistry: Short Review and Recent Literature
National Science Foundation: Feature "Going Live with Click Chemistry"
* ttp://pubs.acs.org/cen/news/85/i43/8543notw8.html Chemical and Engineering News: Feature "Copper-free Click Chemistry"
Metal-free click chemistry review
Click Chemistry
''Chem Soc Rev''
themed issue highlighting the latest applications of click chemistry, guest edited by M. G. Finn and Valery Fokin. Published by the
The Scripps Research Institute
Scripps Research is a nonprofit American medical research facility that focuses on research and education in the biomedical sciences. Headquartered in San Diego, California, the institute has over 170 laboratories employing 2,100 scientists, tec ...
in 2001. The paper argued that synthetic chemistry could emulate the way nature constructs complex molecules, using efficient reactions to join together simple, non-toxic building blocks.
The term "click chemistry" was coined in 1998 by Sharpless' wife, Jan Dueser, who found the simplicity of this approach to chemical synthesis akin to clicking together Lego blocks. In fact, the simplicity of click chemistry represented a paradigm shift in synthetic chemistry, and has had significant impact in many industries, especially pharmaceutical development. In 2022, the Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
was jointly awarded to Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless, "for the development of click chemistry and bioorthogonal chemistry".
Principles
For a reaction to be considered a click reaction, it must satisfy certain characteristics: * modularity * insensitivity to solvent parameters * highchemical yield
In chemistry, yield, also known as reaction yield or chemical yield, refers to the amount of product obtained in a chemical reaction. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis ...
s
* insensitivity towards oxygen and water
* regiospecificity and stereospecificity
* a large thermodynamic driving force (>20 kcal/ mol) to favor a reaction with a single reaction product. A distinct exothermic reaction makes a reactant "spring-loaded".
The process would preferably:
* have simple reaction conditions
* use readily available starting materials and reagents
* use no solvent or use a solvent that is benign or easily removed (preferably water)
* provide simple product isolation by non-chromatographic methods ( crystallisation or distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
)
* have high atom economy
Atom economy (atom efficiency/percentage) is the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced. The simplest definition was introduced by Barry Trost in 1991 and is equal to the rati ...
.
Many of the click chemistry criteria are subjective, and even if measurable and objective criteria could be agreed upon, it is unlikely that any reaction will be perfect for every situation and application. However, several reactions have been identified that fit the concept better than others:
* +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
s, such as the Huisgen 1,3-dipolar cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Ka ...
, in particular the Cu(I)-catalyzed stepwise variant, are often referred to simply as Click reactions
* Thiol-ene reactionLowe, A. B. ''Polymer Chemistry'' 2010, 1 (1), 17–36. DOI: /pubs.rsc.org/en/Content/ArticleLanding/2010/PY/b9py00216b#!divAbstract 10.1039/B9PY00216B/ref>
* Diels-Alder reaction and inverse electron demand Diels-Alder reaction
* +1cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
s between isonitriles (isocyanides) and tetrazines
* nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
especially to small strained rings like epoxy
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide fun ...
and aziridines
* carbonyl-chemistry-like formation of urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
s but not reactions of the aldol type due to low thermodynamic driving force.
* addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
s to carbon-carbon double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s like dihydroxylation
Dihydroxylation is the process by which an alkene is converted into a vicinal (chemistry), vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal ...
or the alkynes in the thiol-yne reaction.
* Sulfur (VI) Fluoride exchange
Specific Click Reactions
Copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)
The classic click reaction is the copper-catalyzed reaction of anazide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
with an alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
to form a 5-membered heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
ring: a Cu(I)-catalyzed azide-alkyne cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
(CuAAC). The first triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial Isomer, isomerism, depending on the positioning of the nitrogen atoms w ...
synthesis, from diethyl acetylenedicarboxylate and phenyl azide, was reported by Arthur Michael
Arthur Michael (August 7, 1853 – February 8, 1942) was an American organic chemist who is best known for the Michael reaction.
Life
Arthur Michael was born into a wealthy family in Buffalo, New York on August 7, 1853, the son of John and Clara ...
in 1893.L. Liang and D. Astruc: "The copper(I)-catalysed alkyne-azide cycloaddition (CuAAC) "click" reaction and its applications. An overview", 2011; 255, 23–24, 2933–2045, p. 2934 Later, in the middle of the 20th century, this family of 1,3-dipolar cycloadditions took on Rolf Huisgen
Rolf Huisgen (; 13 June 1920 – 26 March 2020) was a German chemist. His importance in synthetic organic chemistry extends to the enormous influence he had in post-war chemistry departments in Germany and Austria, due to a large number of his ...
's name after his studies of their reaction kinetics and conditions.
 The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery V. Fokin and K. Barry Sharpless at the Scripps Research Institute in
The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery V. Fokin and K. Barry Sharpless at the Scripps Research Institute in California
California () is a U.S. state, state in the Western United States that lies on the West Coast of the United States, Pacific Coast. It borders Oregon to the north, Nevada and Arizona to the east, and shares Mexico–United States border, an ...
and Morten Meldal in the Carlsberg Laboratory
The Carlsberg Research Laboratory is a private scientific research center in Copenhagen, Denmark under the Carlsberg Foundation. It was founded in 1875 by J. C. Jacobsen, the founder of the Carlsberg brewery, with the purpose of advancing bioche ...
, Denmark. The copper-catalyzed version of this reaction gives only the 1,4-isomer, whereas Huisgen's non-catalyzed 1,3-dipolar cycloaddition gives both the 1,4- and 1,5-isomers, is slow, and requires a temperature of 100 degrees Celsius.
 Moreover, this copper-catalyzed "click" does not require ligands on the metal, although accelerating ligands such as tris(triazolyl)methyl amine ligands with various substituents have been reported and used with success in aqueous solution. Other ligands such as PPh3 and TBIA can also be used, even though PPh3 is liable to Staudinger ligation with the azide substituent. Cu2O in water at room temperature was found also to catalyze the same reaction in 15 minutes with 91% yield.
The first reaction mechanism proposed included one catalytic copper atom; but isotope, kinetic, and other studies have suggested a dicopper mechanism may be more relevant. Even though this reaction proceeds effectively at biological conditions, copper in this range of dosage is cytotoxic. Solutions to this problem have been presented, such as using water-soluble ligands on the copper to enhance cell penetration of the catalyst and thereby reduce the dosage needed,Brotherton, W. S.; Michaels, H. A.; Simmons, J. T.; Clark, R.J.; Dalal, N. S.; Zhu, L. Org. Lett. 2009, 11, 4954. or to use chelating ligands to further increase the effective concentration of Cu(I) and thereby decreasing the actual dosage.Alder, K.; Stein, G.; Finzenhagen, H. Justus Liebigs Ann.Chem 1931, 485, 211.
Although the Cu(I)-catalyzed variant was first reported by Meldal and co-workers for the synthesis of peptidotriazoles on solid support, their conditions were far from the true spirit of click chemistry and were overtaken by the publicly more recognized Sharpless. Meldal and co-workers also chose not to label this reaction type "click chemistry" which allegedly caused their discovery to be largely overlooked by the mainstream chemical society. Fokin and Sharpless independently described it as a reliable catalytic process offering "an unprecedented level of selectivity, reliability, and scope for those organic synthesis endeavors which depend on the creation of covalent links between diverse building blocks".
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.
Moreover, this copper-catalyzed "click" does not require ligands on the metal, although accelerating ligands such as tris(triazolyl)methyl amine ligands with various substituents have been reported and used with success in aqueous solution. Other ligands such as PPh3 and TBIA can also be used, even though PPh3 is liable to Staudinger ligation with the azide substituent. Cu2O in water at room temperature was found also to catalyze the same reaction in 15 minutes with 91% yield.
The first reaction mechanism proposed included one catalytic copper atom; but isotope, kinetic, and other studies have suggested a dicopper mechanism may be more relevant. Even though this reaction proceeds effectively at biological conditions, copper in this range of dosage is cytotoxic. Solutions to this problem have been presented, such as using water-soluble ligands on the copper to enhance cell penetration of the catalyst and thereby reduce the dosage needed,Brotherton, W. S.; Michaels, H. A.; Simmons, J. T.; Clark, R.J.; Dalal, N. S.; Zhu, L. Org. Lett. 2009, 11, 4954. or to use chelating ligands to further increase the effective concentration of Cu(I) and thereby decreasing the actual dosage.Alder, K.; Stein, G.; Finzenhagen, H. Justus Liebigs Ann.Chem 1931, 485, 211.
Although the Cu(I)-catalyzed variant was first reported by Meldal and co-workers for the synthesis of peptidotriazoles on solid support, their conditions were far from the true spirit of click chemistry and were overtaken by the publicly more recognized Sharpless. Meldal and co-workers also chose not to label this reaction type "click chemistry" which allegedly caused their discovery to be largely overlooked by the mainstream chemical society. Fokin and Sharpless independently described it as a reliable catalytic process offering "an unprecedented level of selectivity, reliability, and scope for those organic synthesis endeavors which depend on the creation of covalent links between diverse building blocks".
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.
Strain-promoted azide-alkyne cycloaddition (SPAAC)
The Bertozzi group further developed one of Huisgen's copper-free click reactions to overcome the cytotoxicity of the CuAAC reaction. Instead of using Cu(I) to activate the alkyne, the alkyne is instead introduced in a strained (DIFO), in which the electron-withdrawing, propargylic, gem-fluorines act together with the ring strain to greatly destabilize the alkyne. This destabilization increases the reaction driving force, and the desire of the cycloalkyne to relieve its ring strain. This reaction proceeds as a concerted +2cycloaddition to the triple bond in a cyclooctyne in the same mechanism as the Huisgen 1,3-dipolar cycloaddition. Substituents other than fluorines, such as benzene rings, are also allowed on the cyclooctyne.
This reaction has been used successfully to probe for azides in living systems, even though the reaction rate is somewhat slower than that of the CuAAC. Moreover, because the synthesis of cyclooctynes often gives low yield, probe development for this reaction has not been as rapid as for other reactions. But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO or DBCO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.
This reaction proceeds as a concerted +2cycloaddition to the triple bond in a cyclooctyne in the same mechanism as the Huisgen 1,3-dipolar cycloaddition. Substituents other than fluorines, such as benzene rings, are also allowed on the cyclooctyne.
This reaction has been used successfully to probe for azides in living systems, even though the reaction rate is somewhat slower than that of the CuAAC. Moreover, because the synthesis of cyclooctynes often gives low yield, probe development for this reaction has not been as rapid as for other reactions. But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO or DBCO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.
Strain-promoted alkyne-nitrone cycloaddition (SPANC)
Diaryl-strained-cyclooctynes including dibenzylcyclooctyne (DIBO) have also been used to react with 1,3-nitrones in strain-promoted alkyne-nitrone cycloadditions (SPANC) to yield N-alkylated isoxazolines. Because this reaction is metal-free and proceeds with fast kinetics (k2 as fast as 60 1/Ms, faster than both the CuAAC or the SPAAC) SPANC can be used for live cell labeling. Moreover, substitution on both the carbon and nitrogen atoms of the nitrone dipole, and acyclic and endocyclic nitrones are all tolerated. This large allowance provides a lot of flexibility for nitrone handle or probe incorporation.
However, the isoxazoline product is not as stable as the triazole product of the CuAAC and the SpAAC, and can undergo rearrangements at biological conditions. Regardless, this reaction is still very useful as it has notably fast reaction kinetics.
The applications of this reaction include labeling proteins containing serine as the first residue: the serine is oxidized to aldehyde with NaIO4 and then converted to nitrone with p-methoxybenzenethiol, N-methylhydroxylamine and p-ansidine, and finally incubated with cyclooctyne to give a click product. The SPANC also allows for multiplex labeling.
Because this reaction is metal-free and proceeds with fast kinetics (k2 as fast as 60 1/Ms, faster than both the CuAAC or the SPAAC) SPANC can be used for live cell labeling. Moreover, substitution on both the carbon and nitrogen atoms of the nitrone dipole, and acyclic and endocyclic nitrones are all tolerated. This large allowance provides a lot of flexibility for nitrone handle or probe incorporation.
However, the isoxazoline product is not as stable as the triazole product of the CuAAC and the SpAAC, and can undergo rearrangements at biological conditions. Regardless, this reaction is still very useful as it has notably fast reaction kinetics.
The applications of this reaction include labeling proteins containing serine as the first residue: the serine is oxidized to aldehyde with NaIO4 and then converted to nitrone with p-methoxybenzenethiol, N-methylhydroxylamine and p-ansidine, and finally incubated with cyclooctyne to give a click product. The SPANC also allows for multiplex labeling.
Reactions of strained alkenes
Strained alkenes also utilize strain-relief as a driving force that allows for their participation in click reactions. Trans-cycloalkenes (usually cyclooctenes) and other strained alkenes such as oxanorbornadiene react in click reactions with a number of partners including azides, tetrazines and tetrazoles. These reaction partners can interact specifically with the strained alkene, staying bioorthogonal to endogenous alkenes found in lipids, fatty acids, cofactors and other natural products.Alkene and azide +2cycloaddition
Oxanorbornadiene (or another activated alkene) reacts with azides, giving triazoles as a product. However, these product triazoles are not aromatic as they are in the CuAAC or SPAAC reactions, and as a result are not as stable. The activated double bond in oxanobornadiene makes a triazoline intermediate that subsequently spontaneously undergoes a retro Diels-alder reaction to release furan and give 1,2,3- or 1,4,5-triazoles. Even though this reaction is slow, it is useful because oxabornodiene is relatively simple to synthesize. The reaction is not, however, entirely chemoselective.Alkene and tetrazine inverse-demand Diels-Alder
 Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro +2 cycloaddition (see figure). Like the other reactions of the trans-cyclooctene, ring strain release is a driving force for this reaction. Thus, three-membered and four-membered cycloalkenes, due to their high ring strain, make ideal alkene substrates.
Similar to other +2cycloadditions, electron-donating substituents on the dienophile and electron-withdrawing substituents on the diene accelerate the inverse-demand Diels-Alder. The diene, the tetrazine, by virtue of having the additional nitrogens, is a good diene for this reaction. The dienophile, the activated alkene, can often be attached to electron-donating alkyl groups on target molecules, thus making the dienophile more suitable for the reaction.
Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro +2 cycloaddition (see figure). Like the other reactions of the trans-cyclooctene, ring strain release is a driving force for this reaction. Thus, three-membered and four-membered cycloalkenes, due to their high ring strain, make ideal alkene substrates.
Similar to other +2cycloadditions, electron-donating substituents on the dienophile and electron-withdrawing substituents on the diene accelerate the inverse-demand Diels-Alder. The diene, the tetrazine, by virtue of having the additional nitrogens, is a good diene for this reaction. The dienophile, the activated alkene, can often be attached to electron-donating alkyl groups on target molecules, thus making the dienophile more suitable for the reaction.
Alkene and tetrazole ''photoclick'' reaction
The tetrazole-alkene "photoclick" reaction is another dipolar addition that Huisgen first introduced in the late 1960s ChemBioChem 2007, 8, 1504. (68) Clovis, J. S.; Eckell, A.; Huisgen, R.; Sustmann, R. Chem. Ber. 1967, 100, 60.) Tetrazoles with amino or styryl groups that can be activated by UV light at 365 nm (365 does not damage cells) react quickly (so that the UV light does not have to be on for a long time, usually around 1–4 minutes) to make fluorogenic pyrazoline products. This reaction scheme is well suited for the purpose of labeling in live cells, because UV light at 365 nm damages cells minimally. Moreover, the reaction proceeds quickly, so that the UV light can be administered for short durations. Quantum yields for short wavelength UV light can be higher than 0.5. This allows tetrazoles to be used wavelength selectively in combination with another photoligation reaction, where at the short wavelength the tetrazole ligation reaction proceeds nearly exclusively and at longer wavelength another reaction (ligation via o-quinodimethanes) proceeds exclusively. Finally, the non-fluorogenic reactants give rise to a fluorogenic product, equipping the reaction with a built-in spectrometry handle. Both tetrazoles and the alkene groups have been incorporated as protein handles as unnatural amino acids, but this benefit is not unique. Instead, the photoinducibility of the reaction makes it a prime candidate for spatiotemporal specificity in living systems. Challenges include the presence of endogenous alkenes, though usually cis (as in fatty acids) they can still react with the activated tetrazole.Applications
The criteria for click reactions are designed to make the chemistry biocompatible, for applications like isolating and targeting molecules in complex biological environments. In such environments, products accordingly need to be physiologically stable and any byproducts need to be non-toxic (for ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, an ...
'' systems).
In many applications, click reactions join a biomolecule
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids ...
and a reporter molecule or other molecular probe, a process called bioconjugation. The possibility of attaching fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
s and other reporter molecules has made click chemistry a very powerful tool for identifying, locating, and characterizing both old and new biomolecules..
One of the earliest and most important methods in bioconjugation was to express a reporter gene, such as the gene green fluorescent protein
The green fluorescent protein (GFP) is a protein that exhibits green fluorescence when exposed to light in the blue to ultraviolet range. The label ''GFP'' traditionally refers to the protein first isolated from the jellyfish ''Aequorea victo ...
(GFP), on the same genetic sequence as a protein of interest. In this way, the protein can be identified in cells and tissues by the green fluorescence. However, this approach comes with several difficulties, as the GFP can affect the ability of the protein to achieve its normal shape or hinder its normal expression and functions. Additionally, using this method, GFP can only be attached to proteins, leaving other important biomolecular classes (nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s, lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing ...
s, carbohydrates
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ma ...
, etc.) out of reach.
To overcome these challenges, chemists have opted to proceed by identifying pairs of bioorthogonal reaction partners, thus allowing the use of small exogenous
In a variety of contexts, exogeny or exogeneity () is the fact of an action or object originating externally. It is the opposite of endogeneity or endogeny, the fact of being influenced from within a system.
Economics
In an economic model, an ...
molecules as biomolecular probes. A fluorophore can be attached to one of these probes to give a fluorescence signal upon binding of the reporter molecule to the target—just as GFP fluoresces when it is expressed with the target.
Now limitations emerge from the chemistry of the probe to its target. In order for this technique to be useful in biological systems, click chemistry must run at or near biological conditions, produce little and (ideally) non-toxic byproducts, have (preferably) single and stable products at the same conditions, and proceed quickly to high yield in one pot. Existing reactions, such as Staudinger ligation and the Huisgen 1,3-dipolar cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Ka ...
, have been modified and optimized for such reaction conditions. Today, research in the field concerns not only understanding and developing new reactions and repurposing and re-understanding known reactions, but also expanding methods used to incorporate reaction partners into living systems, engineering novel reaction partners, and developing applications for bioconjugation.
By developing specific and controllable bioorthogonal reactions, scientists have opened up the possibility of hitting particular targets in complex cell lysates. Recently, scientists have adapted click chemistry for use in live cells, for example using small molecule probes that find and attach to their targets by click reactions. Despite challenges of cell permeability, bioorthogonality, background labeling, and reaction efficiency, click reactions have already proven useful in a new generation of pulldown experiments (in which particular targets can be isolated using, for instance, reporter molecules which bind to a certain column), and fluorescence spectrometry (in which the fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
is attached to a target of interest and the target quantified or located). More recently, novel methods have been used to incorporate click reaction partners onto and into biomolecule
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids ...
s, including the incorporation of unnatural amino acids containing reactive groups into proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
and the modification of nucleotides
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
. These techniques represent a part of the field of chemical biology
Chemical biology is a scientific discipline between the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and m ...
, in which click chemistry plays a fundamental role by intentionally and specifically coupling modular units to various ends.
Biotech company Shasqi is a company leveraging click chemistry in humans.
Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, biomimetic and molecular machinery applications.
Click Chemistry is a powerful tool to probe for the cellular localization of small molecules. Knowing where a small molecules goes in the cell gives powerful insights into their mechanisms of action. This approach has been used in numerous studies, and discoveries include that salinomycin localizes to lysosomes to initiate ferroptosis in cancer stem cells and that metformin
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of type2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome, ...
derivatives accumulate in mitochondria to chelate copper(II), affecting metabolism and epigenetic changes downstream in inflammatory macrophages.
The commercial potential of click chemistry is great. The fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
rhodamine
Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are rhodamine 6G, Rhodamin WT, Texas Red (Sulforhodamin 101), rhodamine 123, and rhod ...
has been coupled onto norbornene
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carr ...
, and reacted with tetrazine in living systems. In other cases, SPAAC between a cyclooctyne-modified fluorophore and azide-tagged proteins allowed the selection of these proteins in cell lysates.
 Methods for the incorporation of click reaction partners into systems in and ex vivo contribute to the scope of possible reactions. The development of unnatural amino acid incorporation by ribosomes has allowed for the incorporation of click reaction partners as unnatural side groups on these unnatural amino acids. For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid. In another example, "CpK" has a side group including a cyclopropane alpha to an amide bond that serves as a reaction partner to tetrazine in an inverse diels-alder reaction.
Methods for the incorporation of click reaction partners into systems in and ex vivo contribute to the scope of possible reactions. The development of unnatural amino acid incorporation by ribosomes has allowed for the incorporation of click reaction partners as unnatural side groups on these unnatural amino acids. For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid. In another example, "CpK" has a side group including a cyclopropane alpha to an amide bond that serves as a reaction partner to tetrazine in an inverse diels-alder reaction.
 The synthesis of
The synthesis of luciferin
Luciferin () is a generic term for the light-emitting chemical compound, compound found in organisms that generate bioluminescence. Luciferins typically undergo an enzyme-catalyzed reaction with Oxygen, molecular oxygen. The resulting transforma ...
exemplifies another strategy of isolating reaction partners, which is to take advantage of rarely-occurring, natural groups such as the 1,2-aminothiol, which appears only when a cysteine is the final N' amino acid in a protein. Their natural selectivity and relative bioorthogonality is thus valuable in developing probes specific for these tags. The above reaction occurs between a 1,2-aminothiol and a 2-cyanobenzothiazole to make luciferin, which is fluorescent. This luciferin fluorescence can be then quantified by spectrometry following a wash, and used to determine the relative presence of the molecule bearing the 1,2-aminothiol. If the quantification of non-1,2-aminothiol-bearing protein is desired, the protein of interest can be cleaved to yield a fragment with a N' Cys that is vulnerable to the 2-CBT.
Additional applications include:
* two-dimensional gel electrophoresis separation
*preparative organic synthesis of 1,4-substituted triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial Isomer, isomerism, depending on the positioning of the nitrogen atoms w ...
s
*modification of peptide function with triazoles
*modification of natural products and pharmaceuticals
*natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
discovery
*drug discovery
*macrocyclizations using Cu(I) catalyzed triazole couplings
*modification of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s by triazole ligation
*supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
: calixarene
A calixarene is a macrocycle or cyclic oligomer based on a methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry.
Nomenclature
...
s, rotaxane
A rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since ...
s, and catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be se ...
s
*dendrimer
Dendrimers are highly ordered, Branching (polymer chemistry), branched molecules, polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a sph ...
design
*carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
clusters and carbohydrate conjugation by Cu(1) catalyzed triazole ligation reactions
*polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
and biopolymers
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, ...
*surfaces
*material science
*nanotechnology,
* bioconjugation, for example, azidocoumarin, and
*biomaterials
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose – either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. The corresponding f ...
In combination with combinatorial chemistry
Combinatorial chemistry comprises chemical synthesis, chemical synthetic methods that make it possible to prepare a large number (tens to thousands or even millions) of compounds in a single process. These compound library, compound libraries can b ...
, high-throughput screening
High-throughput screening (HTS) is a method for scientific discovery especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling device ...
, and building chemical libraries
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
, click chemistry has hastened new drug discoveries by making each reaction in a multistep synthesis fast, efficient, and predictable.
Technology license
The Scripps Research Institute has a portfolio of click-chemistry patents. Licensees includeInvitrogen
Invitrogen is one of several brands under the Thermo Fisher Scientific corporation. The product line includes various subbrands of biotechnology products, such as machines and consumables for polymerase chain reaction, reverse transcription, ...
, Allozyne, Aileron, Integrated Diagnostics, and the biotech company , a BASF spin-off created to sell products made using click chemistry. Moreover, holds a worldwide exclusive license for the research and diagnostic market for the nucleic acid field.
Fluorescent azides and alkynes are also produced by companies such as Cyandye.
References
External links
Click Chemistry: Short Review and Recent Literature
National Science Foundation: Feature "Going Live with Click Chemistry"
* ttp://pubs.acs.org/cen/news/85/i43/8543notw8.html Chemical and Engineering News: Feature "Copper-free Click Chemistry"
Metal-free click chemistry review
Click Chemistry
''Chem Soc Rev''
themed issue highlighting the latest applications of click chemistry, guest edited by M. G. Finn and Valery Fokin. Published by the
Royal Society of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the ...
{{DEFAULTSORT:Click Chemistry
Organic chemistry