chemical analysis on:
[Wikipedia]
[Google]
[Amazon]
 Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.
Analytical chemistry consists of classical, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.
Analytical chemistry consists of classical, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as
 Analytical chemistry has been important since the early days of chemistry, providing methods for determining which elements and chemicals are present in the object in question. During this period, significant contributions to analytical chemistry included the development of systematic elemental analysis by Justus von Liebig and systematized organic analysis based on the specific reactions of functional groups.
The first instrumental analysis was flame emissive spectrometry developed by Robert Bunsen and Gustav Kirchhoff who discovered rubidium (Rb) and
Analytical chemistry has been important since the early days of chemistry, providing methods for determining which elements and chemicals are present in the object in question. During this period, significant contributions to analytical chemistry included the development of systematic elemental analysis by Justus von Liebig and systematized organic analysis based on the specific reactions of functional groups.
The first instrumental analysis was flame emissive spectrometry developed by Robert Bunsen and Gustav Kirchhoff who discovered rubidium (Rb) and
 Although modern analytical chemistry is dominated by sophisticated instrumentation, the roots of analytical chemistry and some of the principles used in modern instruments are from traditional techniques, many of which are still used today. These techniques also tend to form the backbone of most undergraduate analytical chemistry educational labs.
Although modern analytical chemistry is dominated by sophisticated instrumentation, the roots of analytical chemistry and some of the principles used in modern instruments are from traditional techniques, many of which are still used today. These techniques also tend to form the backbone of most undergraduate analytical chemistry educational labs.

 Mass spectrometry measures mass-to-charge ratio of molecules using
Mass spectrometry measures mass-to-charge ratio of molecules using
 Separation processes are used to decrease the complexity of material mixtures.
Separation processes are used to decrease the complexity of material mixtures.
 The visualization of single molecules, single cells, biological tissues, and nanomaterials is an important and attractive approach in analytical science. Also, hybridization with other traditional analytical tools is revolutionizing analytical science.
The visualization of single molecules, single cells, biological tissues, and nanomaterials is an important and attractive approach in analytical science. Also, hybridization with other traditional analytical tools is revolutionizing analytical science.
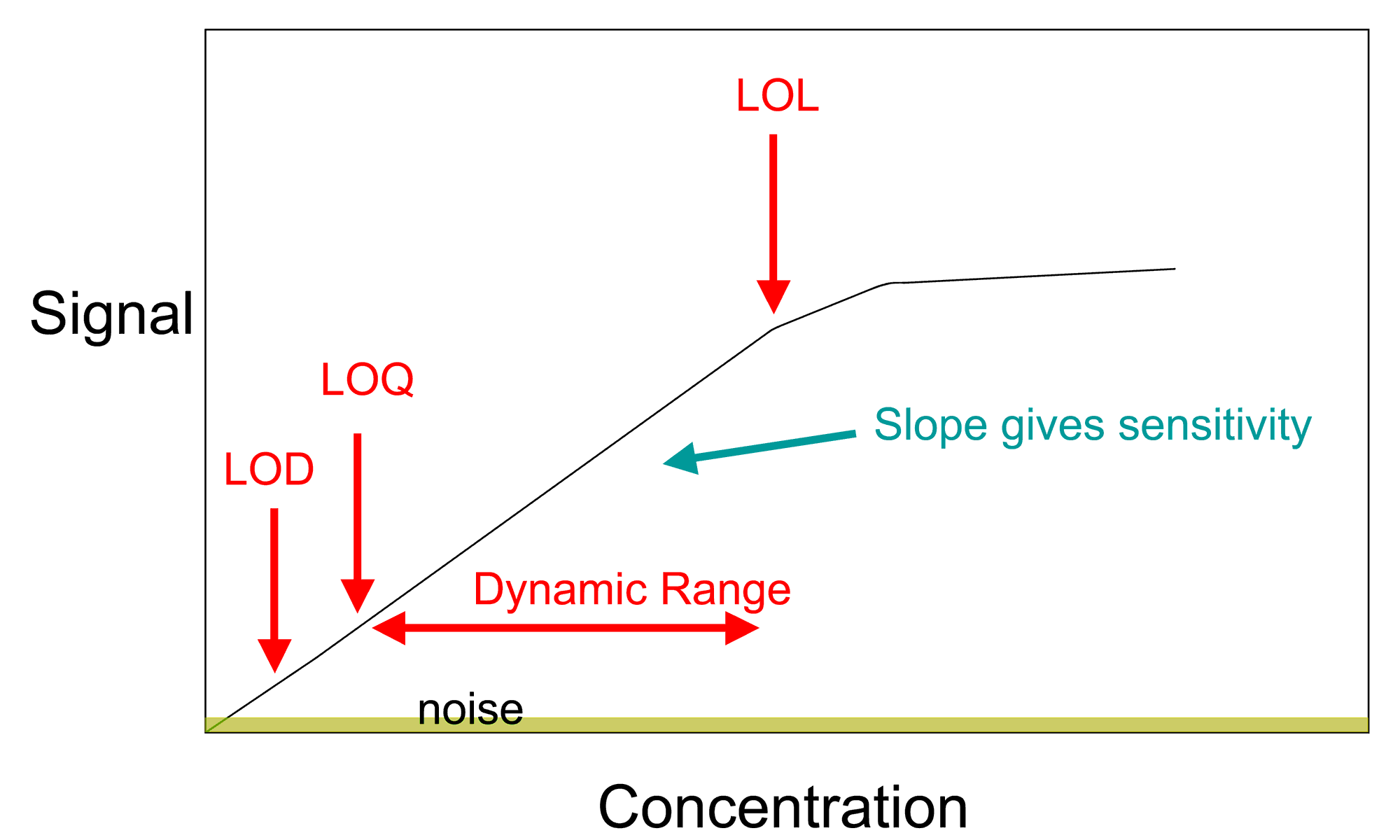 A general method for analysis of concentration involves the creation of a calibration curve. This allows for the determination of the amount of a chemical in a material by comparing the results of an unknown sample to those of a series of known standards. If the concentration of element or compound in a sample is too high for the detection range of the technique, it can simply be diluted in a pure solvent. If the amount in the sample is below an instrument's range of measurement, the method of addition can be used. In this method, a known quantity of the element or compound under study is added, and the difference between the concentration added and the concentration observed is the amount actually in the sample.
A general method for analysis of concentration involves the creation of a calibration curve. This allows for the determination of the amount of a chemical in a material by comparing the results of an unknown sample to those of a series of known standards. If the concentration of element or compound in a sample is too high for the detection range of the technique, it can simply be diluted in a pure solvent. If the amount in the sample is below an instrument's range of measurement, the method of addition can be used. In this method, a known quantity of the element or compound under study is added, and the difference between the concentration added and the concentration observed is the amount actually in the sample.

Infografik
an
animation
showing the progress of analytical chemistry
aas
Atomic Absorption Spectrophotometer {{Authority control Materials science
 Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.
Analytical chemistry consists of classical, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.
Analytical chemistry consists of classical, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with the same instrument and may use light interaction, heat interaction, electric fields or magnetic fields. Often the same instrument can separate, identify and quantify an analyte.
Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools. Analytical chemistry has broad applications to medicine, science, and engineering.
History
 Analytical chemistry has been important since the early days of chemistry, providing methods for determining which elements and chemicals are present in the object in question. During this period, significant contributions to analytical chemistry included the development of systematic elemental analysis by Justus von Liebig and systematized organic analysis based on the specific reactions of functional groups.
The first instrumental analysis was flame emissive spectrometry developed by Robert Bunsen and Gustav Kirchhoff who discovered rubidium (Rb) and
Analytical chemistry has been important since the early days of chemistry, providing methods for determining which elements and chemicals are present in the object in question. During this period, significant contributions to analytical chemistry included the development of systematic elemental analysis by Justus von Liebig and systematized organic analysis based on the specific reactions of functional groups.
The first instrumental analysis was flame emissive spectrometry developed by Robert Bunsen and Gustav Kirchhoff who discovered rubidium (Rb) and caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
(Cs) in 1860.
Most of the major developments in analytical chemistry took place after 1900. During this period, instrumental analysis became progressively dominant in the field. In particular, many of the basic spectroscopic and spectrometric techniques were discovered in the early 20th century and refined in the late 20th century.
The separation sciences follow a similar time line of development and also became increasingly transformed into high performance instruments. In the 1970s many of these techniques began to be used together as hybrid techniques to achieve a complete characterization of samples.
Starting in the 1970s, analytical chemistry became progressively more inclusive of biological questions ( bioanalytical chemistry), whereas it had previously been largely focused on inorganic or small organic molecules. Lasers have been increasingly used as probes and even to initiate and influence a wide variety of reactions. The late 20th century also saw an expansion of the application of analytical chemistry from somewhat academic chemical questions to forensic
Forensic science combines principles of law and science to investigate criminal activity. Through crime scene investigations and laboratory analysis, forensic scientists are able to link suspects to evidence. An example is determining the time and ...
, environmental, industrial and medical
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
questions, such as in histology
Histology,
also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissue (biology), tissues. Histology is the microscopic counterpart to gross anatomy, which looks at large ...
.
Modern analytical chemistry is dominated by instrumental analysis. Many analytical chemists focus on a single type of instrument. Academics tend to either focus on new applications and discoveries or on new methods of analysis. The discovery of a chemical present in blood that increases the risk of cancer would be a discovery that an analytical chemist might be involved in. An effort to develop a new method might involve the use of a tunable laser to increase the specificity and sensitivity of a spectrometric method. Many methods, once developed, are kept purposely static so that data can be compared over long periods of time. This is particularly true in industrial quality assurance (QA), forensic and environmental applications. Analytical chemistry plays an increasingly important role in the pharmaceutical industry where, aside from QA, it is used in the discovery of new drug candidates and in clinical applications where understanding the interactions between the drug and the patient are critical.
Classical methods
 Although modern analytical chemistry is dominated by sophisticated instrumentation, the roots of analytical chemistry and some of the principles used in modern instruments are from traditional techniques, many of which are still used today. These techniques also tend to form the backbone of most undergraduate analytical chemistry educational labs.
Although modern analytical chemistry is dominated by sophisticated instrumentation, the roots of analytical chemistry and some of the principles used in modern instruments are from traditional techniques, many of which are still used today. These techniques also tend to form the backbone of most undergraduate analytical chemistry educational labs.
Qualitative analysis
Qualitative analysis determines the presence or absence of a particular compound, but not the mass or concentration. By definition, qualitative analyses do not measure quantity.Chemical tests
There are numerous qualitative chemical tests, for example, the acid test forgold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
and the Kastle-Meyer test for the presence of blood.
Flame test
Inorganic qualitative analysis generally refers to a systematic scheme to confirm the presence of certain aqueous ions or elements by performing a series of reactions that eliminate a range of possibilities and then confirm suspected ions with a confirming test. Sometimes small carbon-containing ions are included in such schemes. With modern instrumentation, these tests are rarely used but can be useful for educational purposes and in fieldwork or other situations where access to state-of-the-art instruments is not available or expedient.Quantitative analysis
Quantitative analysis is the measurement of the quantities of particular chemical constituents present in a substance. Quantities can be measured by mass (gravimetric analysis) or volume (volumetric analysis).Gravimetric analysis
The gravimetric analysis involves determining the amount of material present by weighing the sample before and/or after some transformation. A common example used in undergraduate education is the determination of the amount of water in a hydrate by heating the sample to remove the water such that the difference in weight is due to the loss of water.Volumetric analysis
Titration involves the gradual addition of a measurable reactant to an exact volume of a solution being analyzed until some equivalence point is reached. Titration is a family of techniques used to determine the concentration of an analyte. Titrating accurately to either the half-equivalence point or the endpoint of a titration allows the chemist to determine the amount of moles used, which can then be used to determine a concentration or composition of the titrant. Most familiar to those who have taken chemistry during secondary education is the acid-base titration involving a color-changing indicator, such as phenolphthalein. There are many other types of titrations, for example, potentiometric titrations or precipitation titrations. Chemists might also create titration curves in order by systematically testing the pH every drop in order to understand different properties of the titrant.Instrumental methods

Spectroscopy
Spectroscopy measures the interaction of the molecules withelectromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
. Spectroscopy consists of many different applications such as atomic absorption spectroscopy, atomic emission spectroscopy, ultraviolet-visible spectroscopy, X-ray spectroscopy, fluorescence spectroscopy, infrared spectroscopy, Raman spectroscopy, dual polarization interferometry, nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a Spectroscopy, spectroscopic technique based on re-orientation of Atomic nucleus, atomic nuclei with non-zero nuclear sp ...
, photoemission spectroscopy, Mössbauer spectroscopy and so on.
Mass spectrometry
 Mass spectrometry measures mass-to-charge ratio of molecules using
Mass spectrometry measures mass-to-charge ratio of molecules using electric
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
and magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
s. In a mass spectrometer, a small amount of sample is ionized and converted to gaseous ions, where they are separated and analyzed according to their mass-to-charge ratios. There are several ionization methods: electron ionization, chemical ionization, electrospray ionization, fast atom bombardment, matrix-assisted laser desorption/ionization, and others. Also, mass spectrometry is categorized by approaches of mass analyzers: magnetic-sector, quadrupole mass analyzer, quadrupole ion trap, time-of-flight, Fourier transform ion cyclotron resonance, and so on.
Electrochemical analysis
Electroanalytical methods measure the potential ( volts) and/or current ( amps) in anelectrochemical cell
An electrochemical cell is a device that either generates electrical energy from chemical reactions in a so called galvanic cell, galvanic or voltaic cell, or induces chemical reactions (electrolysis) by applying external electrical energy in an ...
containing the analyte. These methods can be categorized according to which aspects of the cell are controlled and which are measured. The four main categories are potentiometry (the difference in electrode potentials is measured), coulometry (the transferred charge is measured over time), amperometry (the cell's current is measured over time), and voltammetry (the cell's current is measured while actively altering the cell's potential).
Potentiometry measures the cell's potential, coulometry measures the cell's current, and voltammetry measures the change in current when cell potential changes.
Thermal analysis
Calorimetry and thermogravimetric analysis measure the interaction of a material andheat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
.
Separation
 Separation processes are used to decrease the complexity of material mixtures.
Separation processes are used to decrease the complexity of material mixtures. Chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
, electrophoresis and field flow fractionation are representative of this field.
Chromatographic assays
Chromatography can be used to determine the presence of substances in a sample as different components in a mixture have different tendencies to adsorb onto the stationary phase or dissolve in the mobile phase. Thus, different components of the mixture move at different speed. Different components of a mixture can therefore be identified by their respective R''ƒ'' values, which is the ratio between the migration distance of the substance and the migration distance of the solvent front during chromatography. In combination with the instrumental methods, chromatography can be used in quantitative determination of the substances. Chromatography separates the analyte from the rest of the sample so that it may be measured without interference from other compounds. There are different types of chromatography that differ from the media they use to separate the analyte and the sample. In Thin-layer chromatography, the analyte mixture moves up and separates along the coated sheet under the volatile mobile phase. InGas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include t ...
, gas separates the volatile analytes. A common method for chromatography using liquid as a mobile phase is High-performance liquid chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can origin ...
.
Hybrid techniques
Combinations of the above techniques produce a "hybrid" or "hyphenated" technique. Several examples are in popular use today and new hybrid techniques are under development. For example, gas chromatography-mass spectrometry, gas chromatography- infrared spectroscopy, liquid chromatography-mass spectrometry, liquid chromatography- NMR spectroscopy, liquid chromatography-infrared spectroscopy, and capillary electrophoresis-mass spectrometry. Hyphenated separation techniques refer to a combination of two (or more) techniques to detect and separate chemicals from solutions. Most often the other technique is some form ofchromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
. Hyphenated techniques are widely used in chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
and biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
. A slash is sometimes used instead of hyphen
The hyphen is a punctuation mark used to join words and to separate syllables of a single word. The use of hyphens is called hyphenation.
The hyphen is sometimes confused with dashes (en dash , em dash and others), which are wider, or with t ...
, especially if the name of one of the methods contains a hyphen itself.
Microscopy
 The visualization of single molecules, single cells, biological tissues, and nanomaterials is an important and attractive approach in analytical science. Also, hybridization with other traditional analytical tools is revolutionizing analytical science.
The visualization of single molecules, single cells, biological tissues, and nanomaterials is an important and attractive approach in analytical science. Also, hybridization with other traditional analytical tools is revolutionizing analytical science. Microscopy
Microscopy is the technical field of using microscopes to view subjects too small to be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of microscopy: optical mic ...
can be categorized into three different fields: optical microscopy, electron microscopy, and scanning probe microscopy
Scanning probe microscopy (SPM) is a branch of microscopy that forms images of surfaces using a physical probe that scans the specimen. SPM was founded in 1981, with the invention of the scanning tunneling microscope, an instrument for imaging ...
. Recently, this field is rapidly progressing because of the rapid development of the computer and camera industries.
Lab-on-a-chip
Devices that integrate (multiple) laboratory functions on a single chip of only millimeters to a few square centimeters in size and that are capable of handling extremely small fluid volumes down to less than picoliters.Errors
Error can be defined as numerical difference between observed value and true value. The experimental error can be divided into two types, systematic error and random error. Systematic error results from a flaw in equipment or the design of an experiment while random error results from uncontrolled or uncontrollable variables in the experiment. In error the true value and observed value in chemical analysis can be related with each other by the equation : where * is the absolute error. * is the true value. * is the observed value. An error of a measurement is an inverse measure of accurate measurement, i.e. smaller the error greater the accuracy of the measurement. Errors can be expressed relatively. Given the relative error(): : The percent error can also be calculated: : If we want to use these values in a function, we may also want to calculate the error of the function. Let be a function with variables. Therefore, the propagation of uncertainty must be calculated in order to know the error in : :Standards
Standard curve
 A general method for analysis of concentration involves the creation of a calibration curve. This allows for the determination of the amount of a chemical in a material by comparing the results of an unknown sample to those of a series of known standards. If the concentration of element or compound in a sample is too high for the detection range of the technique, it can simply be diluted in a pure solvent. If the amount in the sample is below an instrument's range of measurement, the method of addition can be used. In this method, a known quantity of the element or compound under study is added, and the difference between the concentration added and the concentration observed is the amount actually in the sample.
A general method for analysis of concentration involves the creation of a calibration curve. This allows for the determination of the amount of a chemical in a material by comparing the results of an unknown sample to those of a series of known standards. If the concentration of element or compound in a sample is too high for the detection range of the technique, it can simply be diluted in a pure solvent. If the amount in the sample is below an instrument's range of measurement, the method of addition can be used. In this method, a known quantity of the element or compound under study is added, and the difference between the concentration added and the concentration observed is the amount actually in the sample.
Internal standards
Sometimes an internal standard is added at a known concentration directly to an analytical sample to aid in quantitation. The amount of analyte present is then determined relative to the internal standard as a calibrant. An ideal internal standard is an isotopically enriched analyte which gives rise to the method of isotope dilution.Standard addition
The method of standard addition is used in instrumental analysis to determine the concentration of a substance ( analyte) in an unknown sample by comparison to a set of samples of known concentration, similar to using a calibration curve. Standard addition can be applied to most analytical techniques and is used instead of a calibration curve to solve the matrix effect problem.Signals and noise
One of the most important components of analytical chemistry is maximizing the desired signal while minimizing the associatednoise
Noise is sound, chiefly unwanted, unintentional, or harmful sound considered unpleasant, loud, or disruptive to mental or hearing faculties. From a physics standpoint, there is no distinction between noise and desired sound, as both are vibrat ...
. The analytical figure of merit is known as the signal-to-noise ratio
Signal-to-noise ratio (SNR or S/N) is a measure used in science and engineering that compares the level of a desired signal to the level of background noise. SNR is defined as the ratio of signal power to noise power, often expressed in deci ...
(S/N or SNR).
Noise can arise from environmental factors as well as from fundamental physical processes.
Thermal noise
Thermal noise results from the motion of charge carriers (usually electrons) in an electrical circuit generated by their thermal motion. Thermal noise is white noise meaning that the power spectral density is constant throughout thefrequency spectrum
In signal processing, the power spectrum S_(f) of a continuous time signal x(t) describes the distribution of power into frequency components f composing that signal. According to Fourier analysis, any physical signal can be decomposed int ...
.
The root mean square
In mathematics, the root mean square (abbrev. RMS, or rms) of a set of values is the square root of the set's mean square.
Given a set x_i, its RMS is denoted as either x_\mathrm or \mathrm_x. The RMS is also known as the quadratic mean (denote ...
value of the thermal noise in a resistor is given by
:
where ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
, ''T'' is the temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
, ''R'' is the resistance, and is the bandwidth of the frequency .
Shot noise
Shot noise is a type of electronic noise that occurs when the finite number of particles (such aselectron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in an electronic circuit or photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s in an optical device) is small enough to give rise to statistical fluctuations in a signal.
Shot noise is a Poisson process, and the charge carriers that make up the current follow a Poisson distribution. The root mean square current fluctuation is given by
:
where ''e'' is the elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
and ''I'' is the average current. Shot noise is white noise.
Flicker noise
Flicker noise is electronic noise with a 1/''ƒ'' frequency spectrum; as ''f'' increases, the noise decreases. Flicker noise arises from a variety of sources, such as impurities in a conductive channel, generation, and recombination noise in atransistor
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch electrical signals and electric power, power. It is one of the basic building blocks of modern electronics. It is composed of semicondu ...
due to base current, and so on. This noise can be avoided by modulation
Signal modulation is the process of varying one or more properties of a periodic waveform in electronics and telecommunication for the purpose of transmitting information.
The process encodes information in form of the modulation or message ...
of the signal at a higher frequency, for example, through the use of a lock-in amplifier.
Environmental noise

Environmental noise
Environmental noise is an accumulation of noise pollution that occurs outside. This noise can be caused by transport, industrial, and Sport, recreational activities.
Noise is frequently described as 'unwanted sound'. Within this context, envir ...
arises from the surroundings of the analytical instrument. Sources of electromagnetic noise are power lines, radio and television stations, wireless devices, compact fluorescent lamps and electric motor
An electric motor is a machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a electromagnetic coil, wire winding to gene ...
s. Many of these noise sources are narrow bandwidth and, therefore, can be avoided. Temperature and vibration isolation
''Vibration isolation'' is the prevention of transmission of vibration from one component of a system to others parts of the same system, as in Building, buildings or mechanical systems. Vibration is undesirable in many domains, primarily engineere ...
may be required for some instruments.
Noise reduction
Noise reduction can be accomplished either incomputer hardware
Computer hardware includes the physical parts of a computer, such as the central processing unit (CPU), random-access memory (RAM), motherboard, computer data storage, graphics card, sound card, and computer case. It includes external devices ...
or software
Software consists of computer programs that instruct the Execution (computing), execution of a computer. Software also includes design documents and specifications.
The history of software is closely tied to the development of digital comput ...
. Examples of hardware noise reduction are the use of shielded cable, analog filter
Analogue Filter (signal processing), filters are a basic building block of signal processing much used in electronics. Amongst their many applications are the separation of an audio signal before application to bass (music), bass, mid-range sp ...
ing, and signal modulation. Examples of software noise reduction are digital filter
In signal processing, a digital filter is a system that performs mathematical operations on a Sampling (signal processing), sampled, discrete-time signal to reduce or enhance certain aspects of that signal. This is in contrast to the other ma ...
ing, ensemble average, boxcar average, and correlation
In statistics, correlation or dependence is any statistical relationship, whether causal or not, between two random variables or bivariate data. Although in the broadest sense, "correlation" may indicate any type of association, in statistics ...
methods.
Applications
Analytical chemistry has applications including inforensic science
Forensic science combines principles of law and science to investigate criminal activity. Through crime scene investigations and laboratory analysis, forensic scientists are able to link suspects to evidence. An example is determining the time and ...
, bioanalysis, clinical analysis, environmental analysis, and materials analysis. Analytical chemistry research is largely driven by performance (sensitivity, detection limit, selectivity, robustness, dynamic range, linear range, accuracy, precision, and speed), and cost (purchase, operation, training, time, and space). Among the main branches of contemporary analytical atomic spectrometry, the most widespread and universal are optical and mass spectrometry. In the direct elemental analysis of solid samples, the new leaders are laser-induced breakdown and laser ablation mass spectrometry, and the related techniques with transfer of the laser ablation products into inductively coupled plasma. Advances in design of diode lasers and optical parametric oscillators promote developments in fluorescence and ionization spectrometry and also in absorption techniques where uses of optical cavities for increased effective absorption pathlength are expected to expand. The use of plasma- and laser-based methods is increasing. An interest towards absolute (standardless) analysis has revived, particularly in emission spectrometry.
Great effort is being put into shrinking the analysis techniques to chip size. Although there are few examples of such systems competitive with traditional analysis techniques, potential advantages include size/portability, speed, and cost. (micro total analysis system (μTAS) or lab-on-a-chip). Microscale chemistry reduces the amounts of chemicals used.
Many developments improve the analysis of biological systems. Examples of rapidly expanding fields in this area are genomics, DNA sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, thymine, cytosine, and guanine. The ...
and related research in genetic fingerprinting and DNA microarray; proteomics
Proteomics is the large-scale study of proteins. Proteins are vital macromolecules of all living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replicatio ...
, the analysis of protein concentrations and modifications, especially in response to various stressors, at various developmental stages, or in various parts of the body, metabolomics
Metabolomics is the scientific study of chemical processes involving metabolites, the small molecule substrates, intermediates, and products of cell metabolism. Specifically, metabolomics is the "systematic study of the unique chemical fingerpri ...
, which deals with metabolites; transcriptomics, including mRNA and associated fields; lipidomics - lipids and its associated fields; peptidomics - peptides and its associated fields; and metallomics, dealing with metal concentrations and especially with their binding to proteins and other molecules.
Analytical chemistry has played a critical role in the understanding of basic science to a variety of practical applications, such as biomedical applications, environmental monitoring
Environmental monitoring is the processes and activities that are done to characterize and describe the state of the environment. It is used in the preparation of environmental impact assessments, and in many circumstances in which human activit ...
, quality control of industrial manufacturing, forensic science, and so on.
The recent developments in computer automation and information technologies have extended analytical chemistry into a number of new biological fields. For example, automated DNA sequencing machines were the basis for completing human genome projects leading to the birth of genomics. Protein identification and peptide sequencing by mass spectrometry opened a new field of proteomics
Proteomics is the large-scale study of proteins. Proteins are vital macromolecules of all living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replicatio ...
. In addition to automating specific processes, there is effort to automate larger sections of lab testing, such as in companies like Emerald Cloud Lab and Transcriptic.
Analytical chemistry has been an indispensable area in the development of nanotechnology
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing propertie ...
. Surface characterization instruments, electron microscopes and scanning probe microscopes enable scientists to visualize atomic structures with chemical characterizations.
See also
* Calorimeter * Clinical chemistry * Environmental chemistry * Ion beam analysis * List of chemical analysis methods * Important publications in analytical chemistry * List of materials analysis methods * Measurement uncertainty *Metrology
Metrology is the scientific study of measurement. It establishes a common understanding of Unit of measurement, units, crucial in linking human activities. Modern metrology has its roots in the French Revolution's political motivation to stan ...
* Microanalysis
* Nuclear reaction analysis
* Quality of analytical results
* Radioanalytical chemistry
* Rutherford backscattering spectroscopy
* Sensory analysis - in the field of Food science
* Virtual instrumentation
* Working range
References
Further reading
* Gurdeep, Chatwal Anand (2008). ''Instrumental Methods of Chemical Analysis'' Himalaya Publishing House (India) * Ralph L. Shriner, Reynold C. Fuson, David Y. Curtin, Terence C. Morill: ''The systematic identification of organic compounds - a laboratory manual'', Verlag Wiley, New York 1980, 6. edition, . * Bettencourt da Silva, R; Bulska, E; Godlewska-Zylkiewicz, B; Hedrich, M; Majcen, N; Magnusson, B; Marincic, S; Papadakis, I; Patriarca, M; Vassileva, E; Taylor, P; Analytical measurement: measurement uncertainty and statistics, 2012, .External links
Infografik
an
animation
showing the progress of analytical chemistry
aas
Atomic Absorption Spectrophotometer {{Authority control Materials science