Cassie's Law on:
[Wikipedia]
[Google]
[Amazon]
Cassie's law, or the Cassie equation, describes the effective
 reflects the relative strength of the interaction between surface tensions at the three phase contact, and is the geometric ratio between the energy gained in forming a unit area of the solid–liquid interface to that required to form a liquid–air interface. However Young's equation only works for ''ideal'' and ''real'' surfaces and in practice most surfaces are microscopically rough.
reflects the relative strength of the interaction between surface tensions at the three phase contact, and is the geometric ratio between the energy gained in forming a unit area of the solid–liquid interface to that required to form a liquid–air interface. However Young's equation only works for ''ideal'' and ''real'' surfaces and in practice most surfaces are microscopically rough.
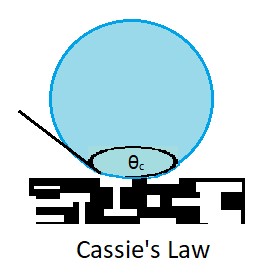
contact angle
The contact angle (symbol ) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interfac ...
θc for a liquid on a chemically heterogeneous surface, i.e. the surface of a composite material
A composite or composite material (also composition material) is a material which is produced from two or more constituent materials. These constituent materials have notably dissimilar chemical or physical properties and are merged to create a ...
consisting of different chemistries, that is, non-uniform throughout. Contact angles are important as they quantify a surface's wettability, the nature of solid-fluid intermolecular interactions. Cassie's law is reserved for when a liquid completely covers both ''smooth'' and ''rough'' heterogeneous surfaces.
More of a rule than a law, the formula found in literature for two materials is;
where and are the contact angles for components 1 with fractional surface area
Area is the measure of a region's size on a surface. The area of a plane region or ''plane area'' refers to the area of a shape or planar lamina, while '' surface area'' refers to the area of an open surface or the boundary of a three-di ...
, and 2 with fractional surface area in the composite material respectively. If there exist more than two materials then the equation is scaled to the general form of;
, with .
Cassie-Baxter
Cassie's law takes on special meaning when the heterogeneous surface is aporous medium
In materials science, a porous medium or a porous material is a material containing pores (voids). The skeletal portion of the material is often called the "matrix" or "frame". The pores are typically filled with a fluid (liquid or gas). The sk ...
. now represents the solid surface area and air gaps, such that the surface is no longer completely wet. Air creates a contact angle of and because = , the equation reduces to:
, which is the Cassie-Baxter equation.
Unfortunately the terms Cassie and Cassie-Baxter are often used interchangeably but they should not be confused. The Cassie-Baxter equation is more common in nature, and focuses on the 'Homogeneous surfaces
The Cassie-Baxter equation is not restricted to only ''chemically'' heterogeneous surfaces, as air within porous homogeneous surfaces will make the ''system'' heterogeneous. However, if the liquid penetrates the grooves, the surface returns to homogeneity and neither of the previous equations can be used. In this case the liquid is in the Wenzel state, governed by a separate equation. Transitions between the Cassie-Baxter state and the Wenzel state can take place when external stimuli such as pressure or vibration are applied to the liquid on the surface.Equation origin
When a liquid droplet interacts with a solid surface, its behaviour is governed by surface tension and energy. The liquid droplet could spread indefinitely or it could sit on the surface like a spherical cap at which point there exists a contact angle. Defining as the free energy change per unit area caused by a liquid spreading, where , are the fractional areas of the two materials on the heterogeneous surface, and and the interfacial tensions between solid, air and liquid. The contact angle for the heterogeneous surface is given by, , with the interfacial tension between liquid and air. The contact angle given by the Young equation is, Thus by substituting the first expression into Young's equation, we arrive at Cassie's law for heterogeneous surfaces,History behind Cassie's law
Young's law
Studies concerning thecontact angle
The contact angle (symbol ) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interfac ...
existing between a liquid and a solid surface began with Thomas Young in 1805. The Young equation

Wenzel state
In 1936 Young's equation was modified by Robert Wenzel to account for rough homogeneous surfaces, and a parameter was introduced, defined as the ratio of the true area of the solid compared to its nominal. Known as the Wenzel equation, shows that the ''apparent contact angle,'' the angle measured at casual inspection, will increase if the surface is roughened. Liquids with contact angle are known to be in the Wenzel state.Cassie-Baxter state
The notion of roughness effecting the contact angle was extended by Cassie and Baxter in 1944 when they focused on porous mediums, where liquid does not penetrate the grooves on rough surface and leaves air gaps. They devised the Cassie-Baxter equation; , sometimes written as where the has become .Cassie's Law
In 1948 Cassie refined this for two materials with different chemistries on both smooth and rough surfaces, resulting in the aforementioned Cassie's lawArguments and inconsistencies
Following the discovery of superhydrophobic surfaces in nature and the growth of their application in industry, the study of contact angles and wetting has been widely reexamined. Some claim that Cassie's equations are more fortuitous than fact with it being argued that emphasis should not be placed on fractional contact areas but actually the behaviour of the liquid at the three phase contact line. They do not argue never using the Wenzel and Cassie-Baxter's equations but that “they should be used with knowledge of their faults”. However the debate continues, as this argument was evaluated and criticised with the conclusion being drawn that contact angles on surfaces ''can'' be described by the Cassie and Cassie-Baxter equations provided the surface fraction and roughness parameters are reinterpreted to take local values appropriate to the droplet. This is why Cassie's ''law'' is actually more of a ''rule.''Examples
It is widely agreed that the water repellency of biological objects is due to the Cassie-Baxter equation. If water has a contact angle between , then the surface is classed as hydrophilic, whereas a surface producing a contact angle between is hydrophobic. In the special cases where the Contact angle is , then it is known as superhydrophobic.Lotus Effect
One example of a superhydrophobic surface in nature is the Lotus leaf. Lotus leaves have a typical contact angle of , ultra low water adhesion due to minimal contact areas, and a self cleaning property which is characterised by the Cassie-Baxter equation. The microscopic architecture of the Lotus leaf means that water will not penetrate nanofolds on the surface, leaving air pockets below. The water droplets become suspended in the Cassie-Baxter state and are able to roll off the leaf picking up dirt as they do so, thus ''cleaning'' the leaf.Feathers
The Cassie–Baxter wetting regime also explains the water repellent features of the pennae (feathers) of a bird. The feather consists of a topography network of 'barbs and barbules' and a droplet that is deposited on a these resides in a solid-liquid-air non-wetting composite state, where tiny air pockets are trapped within.See also
*Wetting
Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. These interactions occur in the presence of either a gaseous phase or ...
* Ultrahydrophobicity
In chemistry and materials science, ultrahydrophobic (or superhydrophobic) surfaces are highly hydrophobic, i.e., extremely difficult to wetting, wet. The contact angles of a water droplet on an ultrahydrophobic material exceed 150°. This is al ...
* Contact angle
The contact angle (symbol ) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interfac ...
* Goniometer
A goniometer is an instrument that either measures an angle or allows an object to be rotated to a precise angular position. The term goniometry derives from two Greek words, γωνία (''gōnía'') 'angle' and μέτρον (''métron'') ' me ...
* Droplet
A drop or droplet is a small column of liquid, bounded completely or almost completely by free surfaces. A drop may form when liquid accumulates at the end of a tube or other surface boundary, producing a hanging drop called a pendant drop. Dro ...
* Lotus effect
The lotus effect refers to self-cleaning properties that are a result of ultrahydrophobicity as exhibited by the leaves of ''Nelumbo'', the lotus flower. Dirt particles are picked up by water droplets due to the micro- and nanoscopic architect ...
References
{{DEFAULTSORT:Cassie's Law Fluid mechanics Surface science