Carbonyl reduction on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 In terms of
In terms of 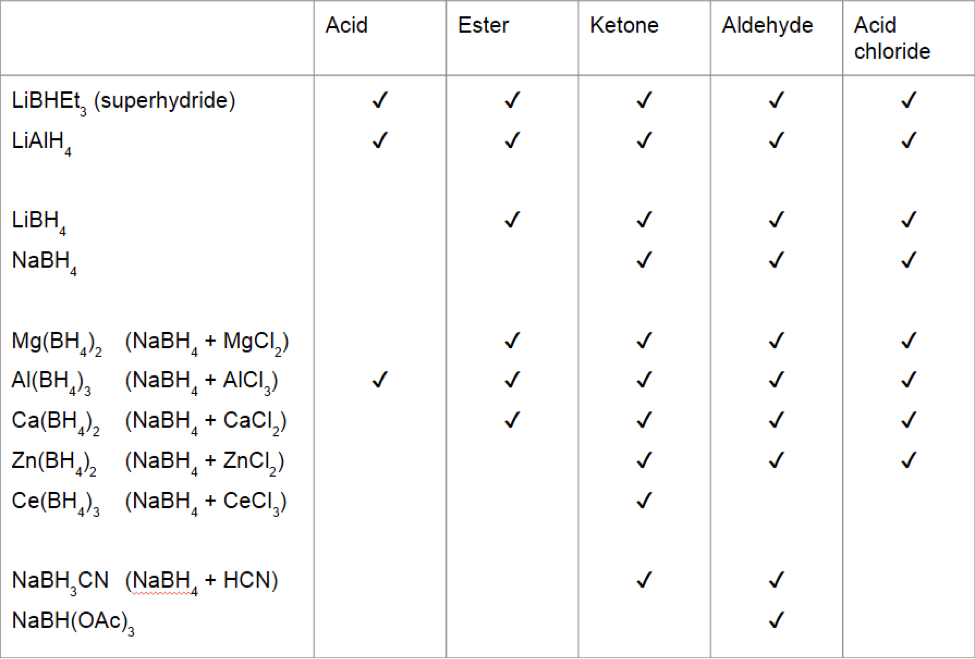
 In the
In the
 The Weinreb amide is reduced via a stable
The Weinreb amide is reduced via a stable  The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
 Aromatic carbonyls are more readily reduced to their respective alkanes than
Aromatic carbonyls are more readily reduced to their respective alkanes than
 Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, carbonyl reduction is the conversion
Conversion or convert may refer to:
Arts, entertainment, and media
* ''The Convert'', a 2023 film produced by Jump Film & Television and Brouhaha Entertainment
* "Conversion" (''Doctor Who'' audio), an episode of the audio drama ''Cyberman''
* ...
of any carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s, aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s, carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s, ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s, and acid halides - some of the most pervasive functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohol
A primary alcohol is an alcohol in which the hydroxy group is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group.
In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary ...
s, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols an ...
. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
Two broad strategies exist for carbonyl reduction. One method, which is favored in industry, uses hydrogen as the reductant. This approach is called hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
and requires metal catalysts. The other broad approach employs stoichiometric reagents that deliver H− and H+ separately. This article focuses on the use of these reagents. Prominent among these reagents are the alkali metal salts of borohydrides and aluminium hydrides.
General considerations
 In terms of
In terms of reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
, metal hydrides effect nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
of hydride to the carbonyl carbon. The ease of addition of hydride to the carbonyl is affected by electrophilicity and bulk of the carbonyl as well as the corresponding electronic and steric properties of the hydride reagent. The result of these trends is that acid halides, ketones, and aldehydes are usually the most readily reduced compounds, while acids and esters require stronger reducing agents. Importantly and characteristically, these hydride reagents generally do not attack C=C bonds.
Several factors contribute to the strength of metal hydride reducing agents. The reducing power of borohydride reagents is affected by the counter ion
160px, cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.
In chemistry, a counterion (sometimes written as "counter ...
, such as Na+ vs Li+ which can activate carbonyls by coordinating to the carbonyl oxygen. Li+ binds to carbonyl oxygen more strongly than does Na+. In the case of tetrahydroaluminates, however, NaAlH4 and LiAlH4 behave similarly. Many metal additives have been investigated. For example, zinc borohydride, nominally , is used for mild selective reduction of aldehydes and ketones in the presence of other reducible groups.
The central metal (usually B vs Al) strongly influences reducing agent's strength. Aluminum hydrides are more nucleophilic and better reducing agents relative to borohydrides. The relatively weak reducer sodium borohydride is typically used for reducing ketones and aldehydes. It tolerates many functional groups (nitro group, nitrile, ester).
In their handling properties, lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthe ...
and sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
(and their derivatives) strongly differ. NaBH4 is far easier to handle than LiAlH4, being air stable for weeks. It can be used with water or ethanol as solvents, whereas LiAlH4 reacts explosively with protic solvents.
Substituents on the boron or aluminium modulate the power, selectivity, and handling properties of these reducing agents. Electron-withdrawing group
An electron-withdrawing group (EWG) is a Functional group, group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effe ...
s such as acetoxy and cyano
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
lower the reducing power such that NaBH(OAc)3 and NaBH3(CN) are weak reducing agents. Electron-donating groups such as alkyl groups enhance the reducing strength. superhydride (lithium triethylborohydride) and L-selectride are strong reductant. They are correspondingly hazardous to handle.
The following table illustrates which carbonyl functional groups can be reduced by which reducing agents (some of these reagents vary in efficacy depending on reaction conditions):

Substrates
Carboxylic acid and esters
Relative to aldehydes and ketones, carboxylic acid are difficult to reduce. Lithium aluminium hydride is typically effective. The first step involves deprotonation of the carboxylic acid. The final step in the reduction of carboxylic acids and esters is hydrolysis of the aluminium alcoxide. Esters (andamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s) are more easily reduced than the parent carboxylic acids. Their reduction affords alcohols and amines, respectively.
The idealized equation for the reduction of an ester by lithium aluminium hydride is:
:
:
Sodium borohydride can, under some circumstances, be used for ester reduction, especially with additives.
Forming aldehydes from carboxylic acid derivatives is challenging because weaker reducing agents (NaBH4) are often very slow at reducing esters and carboxylic acids, whereas stronger reducing agents (LiAlH4) immediately reduce the formed aldehyde to an alcohol.
 In the
In the Fukuyama reduction
The Fukuyama reduction is an organic reaction and an organic reduction in which a thioester is reduced to an aldehyde by a silyl hydride in presence of a catalytic amount of palladium. This reaction was invented in 1990 by Tohru Fukuyama. In the o ...
, a carboxylic acid is first converted to a thioester through addition of a thiol (with a mechanism similar to esterification
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
). The thioester is then reduced to an aldehyde by a silyl hydride with a palladium catalyst.
Acid chlorides to aldehydes
Acid chlorides can be reduced to give aldehydes with sterically hindered hydride donors. The reducing agent DIBAL-H (diisobutylaluminium hydride) is often used for this purpose, although it normally reduces any carbonyl. DIBAL-H can selectively reduce acid chlorides to the aldehyde level if only one equivalent is used at low temperatures. LiAlH(OtBu)3 (formed from LiAlH4 and tBuOH in situ) behaves similarly. The idealized equation for the reduction of an acid chloride to an aldehyde by lithium aluminium hydride is: : The traditional method of forming aldehydes without reducing to alcohols - by using hindered hydrides and reactive carbonyls - is limited by its narrow substrate scope and great dependence on reaction conditions. One workaround to avoid this method is to reduce the carboxylic acid derivative all the way down to an alcohol, then oxidize the alcohol back to an aldehyde. Other alternatives include forming athioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
or a Weinreb amide, then reducing the new species to an aldehyde through the Fukuyama reduction or Weinreb reaction respectively, or using catalytic hydrogenation as in the Rosenmund reaction.
In the Weinreb ketone synthesis, an acyl chloride is first converted to the Weinreb amide, then treated with an organometallic reagent to form a ketone, or lithium aluminum hydride to form an aldehyde:
 The Weinreb amide is reduced via a stable
The Weinreb amide is reduced via a stable chelate
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
, rather than the electrophilic carbonyl that is formed through metal hydride reductions; the chelate is therefore only reduced once, as illustrated below:
 The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
Aldehydes and ketones
Ketones are less reactive than aldehydes, because of greater steric effects, and because the extra alkyl group contributes electron density to the C=O bond, making it less electrophilic. Since, aldehydes reduce more easily than ketones, they require milder reagents and milder conditions. At the other extreme, carboxylic acids, amides, and esters are poorly electrophilic and require strong reducing agents. The idealized equation for the reduction of a ketone by sodium borohydride is: : :Reductive amination
In addition to their reduction to alcohols, aldehydes and ketones can be converted to amines, i.e., reductive amination. Because of its cyano substituent, NaBH3CN is a weak reducer at moderate pH (>4), so it preferentially reduces iminium cations that exist in the presence of carbonyls:α,β-unsaturated carbonyls
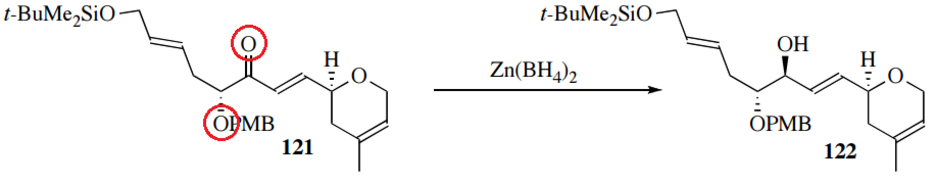
When an α,β-unsaturated carbonyl is reduced, three products can result: an allyl alcohol from simple carbonyl reduction, a saturated ketone or aldehyde resulting from 1,4reduction (also called conjugate reduction), or the saturated alcohol from double reduction. Use of NaBH4 can give any of these results, but InCl3 or NiCl2 catalyze specifically 1,4reductions. Potassium or lithium tri(secbutyl)borohydride sometimes selects 1,4reductions, but can be stymied by steric hindrance. Triphenylphosphinocopper hydride clusters directs catalytic hydrogenation to perform specifically conjugate reduction. To selectively form the allyl alcohol and avoid the 1,4 product, the Luche reduction uses "cerium borohydride" generated in situ from NaBH4 and CeCl3(H2O)7 The hydride source Zn(BH4)2 also shows 1,2 selectivity, as well as greater diastereoselectivity. It does so by coordinating not only to the carbonyl oxygen but also to adjacent atoms:
Hydrogenolysis
A special case of carbonyl reduction entails complete deoxygenation, i.e. hydrogenolysis. This result is often undesirable because it involves defunctionalization. Some reactions for this transformation include theClemmensen reduction
Clemmensen reduction is a chemical reaction described as a Redox, reduction of ketones or aldehydes to alkanes using zinc Amalgam (chemistry), amalgam and concentrated hydrochloric acid (HCl). This reaction is named after Erik Christian Clemmensen ...
(in strongly acidic conditions) and the Wolff–Kishner reduction (in strongly basic conditions), as well as the various modifications of Wolff-Kishner reaction. The Caglioti modification, for instance, uses tosylhydrazone with a hydride donor in milder conditions with no base; the Myers modification substitutes hydrazine with bis(tert-butyldimethylsilyl)-hydrazine, uses milder conditions at room temperature, and is rapid and efficient.
 Aromatic carbonyls are more readily reduced to their respective alkanes than
Aromatic carbonyls are more readily reduced to their respective alkanes than aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
compounds. For example, ketones are reduced to their respective alkyl benzenes by catalytic hydrogenation or by Birch reduction
The Birch reduction or Metal-Ammonia reduction is an organic reaction that is used to convert arenes to Cyclohexa-1,4-diene, 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch (organic chemist), Arthur Birch and i ...
under mild conditions.
Stereoselectivity
Diastereoselective reduction
In the reduction of cyclohexanones, the hydride source can attack axially to produce an equatorial alcohol, or equatorially to produce an axial alcohol. In axial attack (shown in red), the hydride encounters 1,3-diaxial strain. In equatorial attack (shown in blue), the hydride avoids the 1,3-diaxial interaction, but the substrate undergoes unfavorable torsional strain when the newly formed alcohol and added hydrogen atom eclipse each other in the reaction intermediate (as shown in the Newman projection for the axial alcohol). Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
Enantioselective reduction
When asymmetrical ketones are reduced, the resulting secondary alcohol has a chiral center whose can be controlled using chiral transition states or catalysts. In the Evans-Saksena reduction, a nearby alcohol directs the reduction. Well-known carbonyl reductions inasymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
are the Noyori asymmetric hydrogenation (beta-ketoester reduction/Ru/BINAP) and the CBS reduction
CBS Broadcasting Inc., commonly shortened to CBS (an abbreviation of its original name, Columbia Broadcasting System), is an American commercial broadcast television and radio network serving as the flagship property of the CBS Entertainmen ...
(BH3, proline derived chiral catalyst).
History and alternative methods
The Bouveault–Blanc reduction, employing a mixture of sodium metal in the presence of alcohols, was an early method for reduction of carbonyls. It is now largely obsolete. Subsequent to the discovery of the Bouveault–Blanc reduction, many methods were developed, including the major breakthrough of catalytic hydrogenation where H2 serves as the reductant. Salts boron and aluminium hydrides, discovered starting in the 1940s, proved to be highly convenient reagents for carbonyl reduction. In the Meerwein-Ponndorf-Verley reduction, aluminium isopropoxide functions as the hydride source. The status of this reaction has been summarized thusly "the synthetic organic chemist will rarely attempt to use such a conventional technique as the Meerwein−Ponndorf−Verley (MPV) reaction".See also
*Baker's yeast
Baker's yeast is the common name for the strains of yeast commonly used in baking bread and other bakery products, serving as a leavening agent which causes the bread to rise (expand and become lighter and softer) by converting the fermentable ...
, a biotransformation
Biotransformation is the biochemical modification of one chemical compound or a mixture of chemical compounds. Biotransformations can be conducted with whole cells, their lysates, or purified enzymes. Increasingly, biotransformations are effected ...
route for carbonyl reductions.
References
{{Organic reactions Organic redox reactions Nucleophilic addition reactions