Cadmium arsenide on:
[Wikipedia]
[Google]
[Amazon]
Cadmium arsenide ( Cd3 As2) is an inorganic
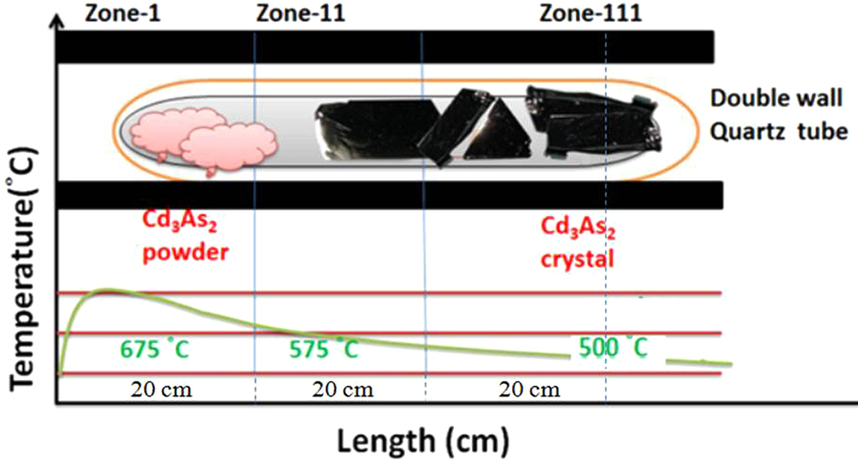 Cadmium arsenide can be prepared as
Cadmium arsenide can be prepared as
National Pollutant Inventory – Cadmium and compounds
{{Arsenides Arsenides Cadmium compounds II-V compounds Semimetals
semimetal
A semimetal is a material with a small energy overlap between the bottom of the Electrical conduction, conduction Electronic band structure, band and the top of the valence band, but they do not overlap in momentum space. According to Band theory ...
in the II-V family. It exhibits the Nernst effect.
Properties
Thermal
Cd3As2 dissociates between 220 and 280 °C according to the reaction :2 Cd3As2(s) → 6 Cd(g) + As4(g) Anenergy barrier
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
was found for the nonstoichiometric vaporization of arsenic due to the irregularity of the partial pressures with temperature. The range of the energy gap is from 0.5 to 0.6 eV. Cd3As2 melts at 716 °C and changes phase at 615 °C/
Phase transition
Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable), α’, α” (metastable), and β. At 593° the polymorphic transition α → β occurs. :α-Cd3As2 ↔ α’-Cd3As2 occurs at ~500 K. :α’-Cd3As2 ↔ α’’-Cd3As2 occurs at ~742 K and is a regular first order phase transition with marked hysteresis loop. :α”-Cd3As2 ↔ β-Cd3As2 occurs at 868 K. Single crystal x-ray diffraction was used to determine the lattice parameters of Cd3As2 between 23 and 700 °C. Transition α → α′ occurs slowly and therefore is most likely an intermediate phase. Transition α′ → α″ occurs much faster than α → α′ and has very small thermalhysteresis
Hysteresis is the dependence of the state of a system on its history. For example, a magnet may have more than one possible magnetic moment in a given magnetic field, depending on how the field changed in the past. Plots of a single component of ...
. This transition results in a change in the fourfold axis of the tetragonal cell, causing crystal twinning
Crystal twinning occurs when two or more adjacent crystals of the same mineral are oriented so that they share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals that are tightl ...
. The width of the loop is independent of the rate of heating although it becomes narrower after several temperature cycles.
Electronic
The compound cadmium arsenide has a lower vapor pressure (0.8 atm) than both cadmium and arsenic separately. Cadmium arsenide does not decompose when it is vaporized and re-condensed. Carrier Concentration in Cd3As2 are usually (1–4)×1018 electrons/cm3. Despite having high carrier concentrations, the electron mobilities are also very high (up to 10,000 cm2/(V·s) at room temperature). In 2014 Cd3As2 was shown to be asemimetal
A semimetal is a material with a small energy overlap between the bottom of the Electrical conduction, conduction Electronic band structure, band and the top of the valence band, but they do not overlap in momentum space. According to Band theory ...
material analogous to graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
that exists in a 3D form that should be much easier to shape into electronic devices. Three-dimensional (3D) topological Dirac semimetals (TDSs) are bulk analogues of graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
that also exhibit non-trivial topology in its electronic structure that shares similarities with topological insulators. Moreover, a TDS can potentially be driven into other exotic phases (such as Weyl semimetals, axion insulators and topological superconductors), Angle-resolved photoemission spectroscopy
Angle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoel ...
revealed a pair of 3D Dirac fermion
In physics, a Dirac fermion is a spin-½ particle (a fermion) which is different from its antiparticle. A vast majority of fermions fall under this category.
Description
In particle physics, all fermions in the standard model have distinct antipar ...
s in Cd3As2. Compared with other 3D TDSs, for example, β-cristobalite and , Cd3As2 is stable and has much higher Fermi velocities. In situ doping was used to tune its Fermi energy.
Conducting
Cadmium arsenide is a II-Vsemiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
showing degenerate n-type semiconductor
N-type, N type or Type N may refer to:
* N-type semiconductor is a key material in the manufacture of transistors and integrated circuits
* An N-type connector is a threaded RF connector used to join coaxial cables
* The MG N-type Magnette was p ...
intrinsic conductivity with a large mobility, low effective mass and highly non parabolic conduction band, or a Narrow-gap semiconductor. It displays an inverted band structure, and the optical energy gap, eg, is less than 0. When deposited by thermal evaporation (deposition), cadmium arsenide displayed the Schottky (thermionic emission
Thermionic emission is the liberation of charged particles from a hot electrode whose thermal energy gives some particles enough kinetic energy to escape the material's surface. The particles, sometimes called ''thermions'' in early literature, a ...
) and Poole–Frenkel effect at high electric fields.
Magnetoresistance
Cadmium Arsenide shows very strong quantum oscillations in resistance even at the relatively high temperature of 100K. This makes it useful for testing cryomagnetic systems as the presence of such a strong signal is a clear indicator of function.Preparation
 Cadmium arsenide can be prepared as
Cadmium arsenide can be prepared as amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
semiconductive glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
. According to Hiscocks and Elliot, the preparation of cadmium arsenide was made from cadmium metal, which had a purity of 6 N from Kock-Light Laboratories Limited. Hoboken supplied β-arsenic with a purity of 99.999%. Stoichiometric proportions of the elements cadmium and arsenic were heated together. Separation was difficult and lengthy due to the ingot
An ingot is a piece of relatively pure material, usually metal, that is Casting, cast into a shape suitable for further processing. In steelmaking, it is the first step among semi-finished casting products. Ingots usually require a second procedu ...
s sticking to the silica and breaking. Liquid encapsulated Stockbarger growth was created. Crystals are pulled from volatile melts in liquid encapsulation. The melt is covered by a layer of inert liquid, usually B2O3, and an inert gas pressure greater than the equilibrium vapor pressure is applied. This eliminates the evaporation from the melt which allows seeding and pulling to occur through the B2O3 layer.
Crystal structure
The unit cell of Cd3As2 is tetragonal. The arsenic ions are cubic close packed and the cadmium ions are tetrahedrally coordinated. The vacant tetrahedral sites provoked research by von Stackelberg and Paulus (1935), who determined the primary structure. Each arsenic ion is surrounded by cadmium ions at six of the eight corners of a distorted cube and the two vacant sites were at the diagonals. The crystalline structure of cadmium arsenide is very similar to that of zinc phosphide (Zn3P2), zinc arsenide (Zn3As2) and cadmium phosphide (Cd3P2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution.Nernst effect
Cadmium arsenide is used ininfrared detector
An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).
The thermal effects of the incident IR radiation can be followed through many temperature depe ...
s using the Nernst effect, and in thin-film dynamic pressure sensor
Pressure measurement is the measurement of an applied force by a fluid (liquid or gas) on a surface. Pressure is typically measured in units of force per unit of surface area. Many techniques have been developed for the measurement of pressur ...
s. It can be also used to make magnetoresistors, and in photodetector
Photodetectors, also called photosensors, are devices that detect light or other forms of electromagnetic radiation and convert it into an electrical signal. They are essential in a wide range of applications, from digital imaging and optical ...
s.
Cadmium arsenide can be used as a dopant
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optics, optical properties. The amount of dopant is typically very low compared to the material b ...
for HgCdTe
Hg1−''x''Cd''x''Te or mercury cadmium telluride (also cadmium mercury telluride, MCT, MerCad Telluride, MerCadTel, MerCaT or CMT) is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning th ...
.
References
External links
National Pollutant Inventory – Cadmium and compounds
{{Arsenides Arsenides Cadmium compounds II-V compounds Semimetals