C2-Symmetric Ligands on:
[Wikipedia]
[Google]
[Amazon]
In homogeneous catalysis, ''C''2-symmetric ligands refer to
 Some classes of ''C''2-symmetric ligands are called privileged ligands, which are ligands that are broadly applicable to multiple catalytic processes, not only a single reaction type.
Some classes of ''C''2-symmetric ligands are called privileged ligands, which are ligands that are broadly applicable to multiple catalytic processes, not only a single reaction type.
(S,S)-DIOP.svg, The ''C''2-symmetric diphosphine DIOP is historically significant.
DuPhos ligands.svg, DuPhos ligands are a class of ''C''2-symmetric ligands for asymmetric hydrogenation.
Oxaliplatin-2D-skeletal.png, Oxaliplatin, containing the ''C''2-symmetric (''R'',''R'')-diaminocyclohexane ligand, is an important anticancer drug.
Jacobsen's catalyst (S,S).png, Jacobsen's epoxidation catalyst is a complex of a ''C''2-symmetric salen-type ligand.
HayashiChiralNBD.svg, ''C''2-symmetric diene ligand.
BOX and PyBOX.png, Both bi- and tridentate bis(oxazoline) ligands are used in
ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
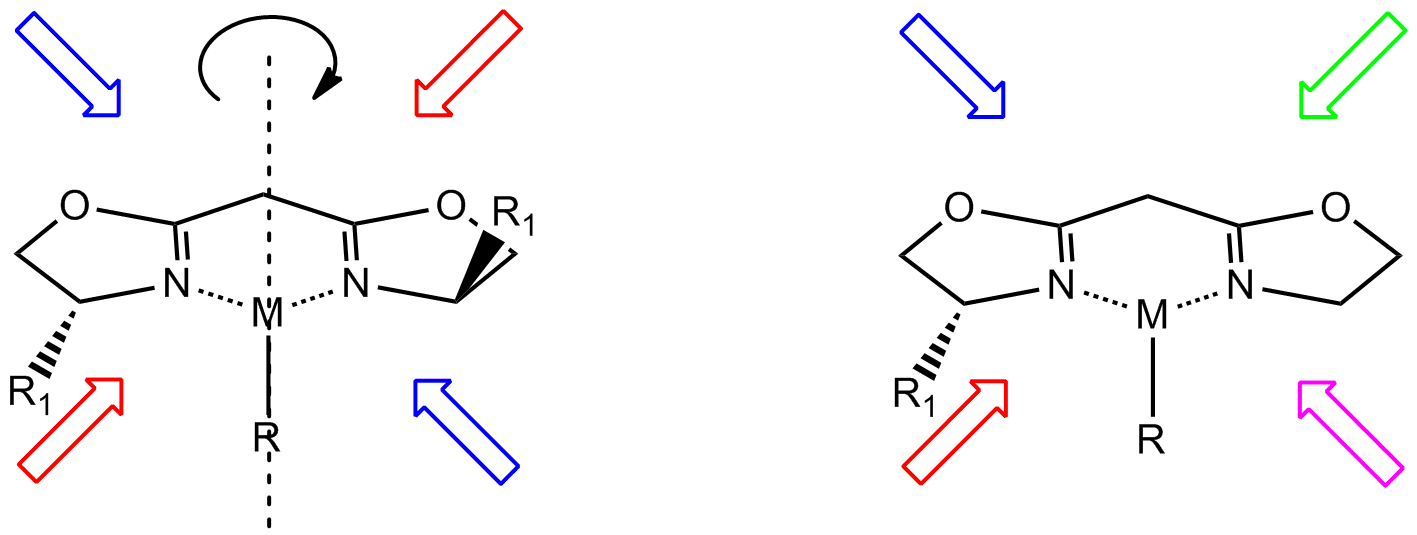
s that lack mirror symmetry but have ''C''2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
. The ''C''2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. ''C''2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including ''C''2-symmetric ligands, combine with metals or other groups to form chiral catalyst
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
s. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Examples
An early ''C''2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 byWilliam S. Knowles
William Standish Knowles (June 1, 1917 – June 13, 2012) was an American chemist. He was born in Taunton, Massachusetts. Knowles was one of the recipients of the 2001 Nobel Prize in Chemistry. He split half the prize with Ryōji Noyori for thei ...
and coworkers of Monsanto Company
The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best known product is Roundup, a glyphosate-based herbicide, developed in the ...
, who shared the 2001 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
. This ligand was used in the industrial production of -DOPA.
:organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
BINAP Enantiomers Structural Formulae V.1.svg, Both enantiomers of BINAP
R-BINOL-2D-skeletal.png, BINOL, another binaphthalene-based ligand
TADDOLgeneric.png, TADDOL
DIPAMP.png, DIPAMP, a diphosphine of historic significance
(DHQ)2PHAL.png, AD-mix α, dihydroquinine
Dihydroquinine, also known as hydroquinine, is an organic compound and as a cinchona alkaloid closely related to quinine. The specific rotation is −148° in ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinki ...
derivative used in Sharpless asymmetric dihydroxylation
Mechanistic concepts
While the presence of any symmetry element within a ligand intended for asymmetric induction might appear counterintuitive, asymmetric induction only requires that the ligand bechiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
(i.e. have no improper rotation axis). Asymmetry (i.e. absence of any symmetry elements) is not required. ''C''2 symmetry improves the enantioselectivity of the complex by reducing the number of unique geometries in the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
s. Steric and kinetic factors then usually favor the formation of a single product.

Chiral fence
: Chiral ligands work by asymmetric induction somewhere along the reaction coordinate. The image to the right illustrates how a chiral ligand may induce an enantioselective reaction. The ligand (in green) has ''C''2 symmetry with its nitrogen, oxygen or phosphorus atoms hugging a central metal atom (in red). In this particular ligand the right side is sticking out and its left side points away. The substrate in this reduction isacetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene ...
and the reagent (in blue) a hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
ion. In absence of the metal and the ligand the ''Re'' face approach of the hydride ion gives the (''S'')-enantiomer and the ''Si'' face approach the (''R'')-enantiomer in equal amounts (a racemic mixture like expected). The ligand and metal presence changes all that. The carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
group will coordinate with the metal and due to the steric bulk of the phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydroge ...
group it will only be able to do so with its ''Si'' face exposed to the hydride ion with in the ideal situation exclusive formation of the (''R'') enantiomer. The re face will simply hit the chiral fence. Note that when the ligand is replaced by its mirror image the other enantiomer will form and that a racemic mixture of ligand will once again yield a racemic product. Also note that if the steric bulk of both carbonyl substituents is very similar the strategy will fail.
Other ''C''2-symmetric complexes
Many ''C''2-symmetric complexes are known. Some arise not from ''C''2-symmetric ligands, but from the orientation or disposition of high symmetry ligands within the coordination sphere of the metal. Notably,EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
and triethylenetetraamine
Triethylenetetramine (TETA and trien), also known as trientine (INN) when used medically, is an organic compound with the formula H2NHCH2CH2NH2sub>2. The pure freebase is a colorless oily liquid, but, like many amines, older samples assume a yel ...
form complexes that are ''C''2-symmetric by virtue of the way the ligands wrap around the metal centers. Two isomers are possible for (indenyl In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related ...
)2MX2, ''C''s- and ''C''2-symmetric. The ''C''2-symmetric complexes are optically stable.
Asymmetric ligands
Ligands containing atomic chirality centers suchasymmetric carbon An asymmetric carbon atom (chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms. Le Bel-van't Hoff rule states that the number of stereoisomers of an organic compound is 2n, where n represents the num ...
, which usually do not have ''C''2-symmetry, remain important in catalysis. Examples include cinchona alkaloid
''Cinchona'' (pronounced or ) is a genus of flowering plants in the family Rubiaceae containing at least 23 species of trees and shrubs. All are native to the tropical Andean forests of western South America. A few species are reportedly nat ...
s and certain phosphoramidite
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these ...
s. P-chiral monophosphines have also been investigated.
See also
*Chiral anion catalysis
Asymmetric counteranion directed catalysis (ACDC) or chiral anion catalysis in enantioselective synthesis is the "induction of enantioselectivity in a reaction proceeding through a cationic intermediate by means of ion pairing with a chiral, e ...
Further reading
* * * * *References
{{DEFAULTSORT:Chiral Ligand Coordination chemistry Stereochemistry Organometallic chemistry Ligands