Bredt's rule on:
[Wikipedia]
[Google]
[Amazon]
In 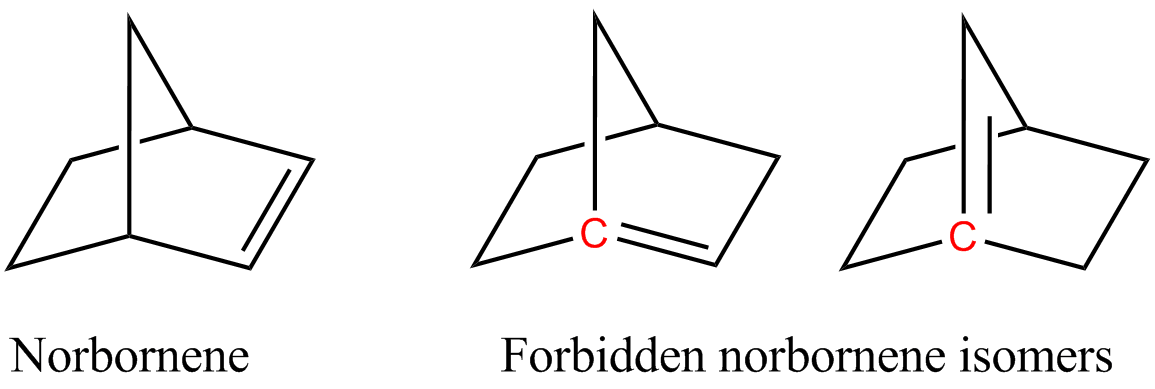 The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. There are a few instances where the anti-Bredt phenomenon is mentioned, but the isolation of these molecules is difficult, so they are typically trapped in situ. In pioneering studies, Wiseman, Keese, Wiberg, and others validated the intermediacy of anti-Bredt olefins beginning in the 1960s. Authors such as Mehta (2002) and Khan (2015) also obtained some possible support for the intermediacy of anti-Bredt olefins. In 2024, Neil Garg and his team demonstrated that the formation of anti-Bredt molecules is possible, even if only as short-lived intermediates, and provided a general synthetic solution to generating and trapping anti-Bredt olefins in cycloadditions.
Bredt's rule results from geometric strain: a double bond at a bridgehead atom necessarily must be ''trans'' in at least one ring. For small rings (fewer than eight atoms), a ''trans'' alkene cannot be achieved without substantial
The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. There are a few instances where the anti-Bredt phenomenon is mentioned, but the isolation of these molecules is difficult, so they are typically trapped in situ. In pioneering studies, Wiseman, Keese, Wiberg, and others validated the intermediacy of anti-Bredt olefins beginning in the 1960s. Authors such as Mehta (2002) and Khan (2015) also obtained some possible support for the intermediacy of anti-Bredt olefins. In 2024, Neil Garg and his team demonstrated that the formation of anti-Bredt molecules is possible, even if only as short-lived intermediates, and provided a general synthetic solution to generating and trapping anti-Bredt olefins in cycloadditions.
Bredt's rule results from geometric strain: a double bond at a bridgehead atom necessarily must be ''trans'' in at least one ring. For small rings (fewer than eight atoms), a ''trans'' alkene cannot be achieved without substantial
Supporting Information
Bicyclo .3.1ndecane-11-one-1-carboxylic acid undergoes decarboxylation on heating to 132 °C, but the similar compound bicyclo .2.1eptan-7-one-1-carboxylic acid remains stable beyond 500 °C, because the decarboxylation proceeds through an anti-Bredt
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, an anti-Bredt molecule is a bridged molecule with a double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
at the bridgehead
In military strategy, a bridgehead (or bridge-head) is the strategically important area of ground around the end of a bridge or other place of possible crossing over a body of water which at time of conflict is sought to be defended or taken over ...
. Bredt's rule is the empirical observation that such molecules only form in large ring systems. For example, two of the following norbornene
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carr ...
isomers violate Bredt's rule, and are too unstable to prepare: The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. There are a few instances where the anti-Bredt phenomenon is mentioned, but the isolation of these molecules is difficult, so they are typically trapped in situ. In pioneering studies, Wiseman, Keese, Wiberg, and others validated the intermediacy of anti-Bredt olefins beginning in the 1960s. Authors such as Mehta (2002) and Khan (2015) also obtained some possible support for the intermediacy of anti-Bredt olefins. In 2024, Neil Garg and his team demonstrated that the formation of anti-Bredt molecules is possible, even if only as short-lived intermediates, and provided a general synthetic solution to generating and trapping anti-Bredt olefins in cycloadditions.
Bredt's rule results from geometric strain: a double bond at a bridgehead atom necessarily must be ''trans'' in at least one ring. For small rings (fewer than eight atoms), a ''trans'' alkene cannot be achieved without substantial
The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. There are a few instances where the anti-Bredt phenomenon is mentioned, but the isolation of these molecules is difficult, so they are typically trapped in situ. In pioneering studies, Wiseman, Keese, Wiberg, and others validated the intermediacy of anti-Bredt olefins beginning in the 1960s. Authors such as Mehta (2002) and Khan (2015) also obtained some possible support for the intermediacy of anti-Bredt olefins. In 2024, Neil Garg and his team demonstrated that the formation of anti-Bredt molecules is possible, even if only as short-lived intermediates, and provided a general synthetic solution to generating and trapping anti-Bredt olefins in cycloadditions.
Bredt's rule results from geometric strain: a double bond at a bridgehead atom necessarily must be ''trans'' in at least one ring. For small rings (fewer than eight atoms), a ''trans'' alkene cannot be achieved without substantial ring
(The) Ring(s) may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
Arts, entertainment, and media Film and TV
* ''The Ring'' (franchise), a ...
and angle strain (the ''p'' orbitals are improperly aligned for a π bond). Bredt's rule also applies to carbocations and, to a lesser degree, free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemi ...
, because these intermediates also prefer a planar geometry with 120° angles and ''sp''2 hybridization. It generally does not apply to hypervalent heteroatoms
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
, although they are commonly written with a formal double bond.
There has been an active research program to seek anti-Bredt molecules, with success quantified in ''S'', the non-bridgehead atom count. The above norbornene system has ''S'' = 5, and Fawcett originally postulated that stability required ''S'' ≥ 9 in bicyclic systems and ''S'' ≥ 11 in tricyclic systems. For bicyclic systems examples now indicate a limit of S ≥ 7, with several such compounds having been prepared. Bridgehead double bonds can be found in some natural products.
Bredt's rule can predict the viability of competing elimination reactions in a bridged system. For example, the metal alkyl complexes usually decompose quickly via beta elimination
Beta (, ; uppercase , lowercase , or cursive ; or ) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Ancient Greek, beta represented the voiced bilabial plosive . In Modern Greek, it represen ...
, but Bredt strain prevents tetranorbornyl complexes from doing so.Supporting Information
Bicyclo .3.1ndecane-11-one-1-carboxylic acid undergoes decarboxylation on heating to 132 °C, but the similar compound bicyclo .2.1eptan-7-one-1-carboxylic acid remains stable beyond 500 °C, because the decarboxylation proceeds through an anti-Bredt
enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
.
Bredt's rule may also prevent a molecule from resonating with certain valence bond isomers. 2-Quinuclidonium does not exhibit the usual reactivity of an amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
, because the iminoether
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between an imidic acid () and an alcohol, with the general formula .
They are also known as imino ethers, since they resemble imines () w ...
tautomer would violate the rule.
Although exceptions to the rule have long been known, in 2024 chemists from University of California, Los Angeles
The University of California, Los Angeles (UCLA) is a public university, public Land-grant university, land-grant research university in Los Angeles, California, United States. Its academic roots were established in 1881 as a normal school the ...
demonstrated a general method to access Anti-Bredt olefins with S ≤ 7 for use in cycloaddition reactions.
See also
*Double bond rule
In chemistry, the double bond rule states that elements with a principal quantum number (''n'') greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form multiple bonds (e.g. double bonds and triple bonds). Do ...
— another geometric-strain constraint on alkenes
* ''trans''-Cyclooctene — smallest unstrained ''trans'' cycloalkene
References
{{Authority control Eponymous chemical rules Physical organic chemistry Stereochemistry