aromatic compound on:
[Wikipedia]
[Google]
[Amazon]
 Aromatic compounds or arenes are
Aromatic compounds or arenes are





 Benzene derivatives have from one to six
Benzene derivatives have from one to six
File:Benzene-Kekule-2D-skeletal.png,

 Nucleophilic aromatic substitution involves displacement of a
Nucleophilic aromatic substitution involves displacement of a

 The
The  The relative binding energies of the three geometries of the benzene dimer can be explained by a balance of quadrupole/quadrupole and
The relative binding energies of the three geometries of the benzene dimer can be explained by a balance of quadrupole/quadrupole and  Hunter ''et al.'' applied a more sophisticated chemical double mutant cycle with a hydrogen-bonded "zipper" to the issue of substituent effects in pi stacking interactions in proteins. However, the authors note that direct interactions with the ring substituents, discussed below, also make important contributions. Indeed, the interplay of these two factors may result in the complicated substituent- and geometry-dependent behavior of pi stacking interactions.
Some experimental and computational evidence suggests that pi stacking interactions are not governed primarily by electrostatic effects..
The relative contributions pi stacking have been borne out by computation. Trends based on electron donating or withdrawing substituents can be explained by exchange-repulsion and dispersion terms.
A molecular torsion balance from an aryl ester with two conformational states. The folded state had a well-defined pi stacking interaction with a T-shaped geometry, whereas the unfolded state had no aryl–aryl interactions. The NMR chemical shifts of the two conformations were distinct and could be used to determine the ratio of the two states, which was interpreted as a measure of intramolecular forces. The authors report that a preference for the folded state is not unique to aryl esters. For example, the cyclohexyl ester favored the folded state more so than the phenyl ester, and the tert-butyl ester favored the folded state by a preference greater than that shown by any aryl ester. This suggests that aromaticity is not a strict requirement for favorable interaction with an aromatic ring.
Other evidence for non-aromatic pi stacking interactions results include critical studies in theoretical chemistry, explaining the underlying mechanisms of empirical observations. Grimme reported that the interaction energies of smaller dimers consisting of one or two rings are very similar for both aromatic and saturated compounds. This finding is of particular relevance to biology, and suggests that the contribution of pi systems to phenomena such as stacked nucleobases may be overestimated. However, it was shown that an increased stabilizing interaction is seen for large aromatic dimers. As previously noted, this interaction energy is highly dependent on geometry. Indeed, large aromatic dimers are only stabilized relative to their saturated counterparts in a sandwich geometry, while their energies are similar in a T-shaped interaction.
Hunter ''et al.'' applied a more sophisticated chemical double mutant cycle with a hydrogen-bonded "zipper" to the issue of substituent effects in pi stacking interactions in proteins. However, the authors note that direct interactions with the ring substituents, discussed below, also make important contributions. Indeed, the interplay of these two factors may result in the complicated substituent- and geometry-dependent behavior of pi stacking interactions.
Some experimental and computational evidence suggests that pi stacking interactions are not governed primarily by electrostatic effects..
The relative contributions pi stacking have been borne out by computation. Trends based on electron donating or withdrawing substituents can be explained by exchange-repulsion and dispersion terms.
A molecular torsion balance from an aryl ester with two conformational states. The folded state had a well-defined pi stacking interaction with a T-shaped geometry, whereas the unfolded state had no aryl–aryl interactions. The NMR chemical shifts of the two conformations were distinct and could be used to determine the ratio of the two states, which was interpreted as a measure of intramolecular forces. The authors report that a preference for the folded state is not unique to aryl esters. For example, the cyclohexyl ester favored the folded state more so than the phenyl ester, and the tert-butyl ester favored the folded state by a preference greater than that shown by any aryl ester. This suggests that aromaticity is not a strict requirement for favorable interaction with an aromatic ring.
Other evidence for non-aromatic pi stacking interactions results include critical studies in theoretical chemistry, explaining the underlying mechanisms of empirical observations. Grimme reported that the interaction energies of smaller dimers consisting of one or two rings are very similar for both aromatic and saturated compounds. This finding is of particular relevance to biology, and suggests that the contribution of pi systems to phenomena such as stacked nucleobases may be overestimated. However, it was shown that an increased stabilizing interaction is seen for large aromatic dimers. As previously noted, this interaction energy is highly dependent on geometry. Indeed, large aromatic dimers are only stabilized relative to their saturated counterparts in a sandwich geometry, while their energies are similar in a T-shaped interaction.
 A more direct approach to modeling the role of aromaticity was taken by Bloom and Wheeler. The authors compared the interactions between benzene and either 2-methylnaphthalene or its non-aromatic isomer, 2-methylene-2,3-dihydronaphthalene. The latter compound provides a means of conserving the number of p-electrons while, however, removing the effects of delocalization. Surprisingly, the interaction energies with benzene are higher for the non-aromatic compound, suggesting that pi-bond localization is favorable in pi stacking interactions. The authors also considered a homodesmotic dissection of benzene into ethylene and 1,3-butadiene and compared these interactions in a sandwich with benzene. Their calculation indicates that the interaction energy between benzene and homodesmotic benzene is higher than that of a benzene dimer in both sandwich and parallel displaced conformations, again highlighting the favorability of localized pi-bond interactions. These results strongly suggest that aromaticity is not required for pi stacking interactions in this model.
Even in light of this evidence, Grimme concludes that pi stacking does indeed exist. However, he cautions that smaller rings, particularly those in T-shaped conformations, do not behave significantly differently from their saturated counterparts, and that the term should be specified for larger rings in stacked conformations which do seem to exhibit a cooperative pi electron effect.
A more direct approach to modeling the role of aromaticity was taken by Bloom and Wheeler. The authors compared the interactions between benzene and either 2-methylnaphthalene or its non-aromatic isomer, 2-methylene-2,3-dihydronaphthalene. The latter compound provides a means of conserving the number of p-electrons while, however, removing the effects of delocalization. Surprisingly, the interaction energies with benzene are higher for the non-aromatic compound, suggesting that pi-bond localization is favorable in pi stacking interactions. The authors also considered a homodesmotic dissection of benzene into ethylene and 1,3-butadiene and compared these interactions in a sandwich with benzene. Their calculation indicates that the interaction energy between benzene and homodesmotic benzene is higher than that of a benzene dimer in both sandwich and parallel displaced conformations, again highlighting the favorability of localized pi-bond interactions. These results strongly suggest that aromaticity is not required for pi stacking interactions in this model.
Even in light of this evidence, Grimme concludes that pi stacking does indeed exist. However, he cautions that smaller rings, particularly those in T-shaped conformations, do not behave significantly differently from their saturated counterparts, and that the term should be specified for larger rings in stacked conformations which do seem to exhibit a cooperative pi electron effect.
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s "with a chemistry typified by benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
.
Aromatic compounds have the following general properties:
* Typically unreactive
* Often non polar and hydrophobic
* High carbon-hydrogen ratio
* Burn with a strong sooty yellow flame, due to high C:H ratio
* Undergo electrophilic substitution reaction
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
s and nucleophilic aromatic substitutions
Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in many reaction types, following both additions and removals, as well as saturation and dearomatization.
Heteroarenes
Heteroarenes are aromatic compounds, where at least one methine or vinylene (-C= or -CH=CH-) group is replaced by aheteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
: oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, or sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
. Examples of non-benzene compounds with aromatic properties are furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
. Approximately half of compounds known in 2000 are described as aromatic to some extent.



Applications
Aromatic compounds are pervasive in nature and industry. Key industrial aromatic hydrocarbons are benzene,toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
, xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are su ...
called BTX. Many biomolecules have phenyl groups including the so-called aromatic amino acid
An aromatic amino acid is an amino acid that includes an aromaticity, aromatic ring.
Among the 20 standard amino acids, histidine, phenylalanine, tryptophan, tyrosine, are classified as aromatic.
Properties and function Optical properties
Ar ...
s.
Benzene ring model


Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, C6H6, is the least complex aromatic hydrocarbon, and it was the first one defined as such. Its bonding nature was first recognized independently by Joseph Loschmidt and August Kekulé
Friedrich August Kekulé, later Friedrich August Kekule von Stradonitz ( , ; 7 September 1829 – 13 July 1896), was a German organic chemist. From the 1850s until his death, Kekulé was one of the most prominent chemists in Europe, especially ...
in the 19th century. Each carbon atom in the hexagonal cycle has four electrons to share. One electron forms a sigma bond with the hydrogen atom, and one is used in covalently bonding to each of the two neighboring carbons. This leaves six electrons, shared equally around the ring in delocalized pi molecular orbitals the size of the ring itself. This represents the equivalent nature of the six carbon-carbon bonds all of bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
1.5. This equivalency can also explained by resonance forms. The electrons are visualized as floating above and below the ring, with the electromagnetic fields they generate acting to keep the ring flat.
The circle symbol for aromaticity was introduced by Sir Robert Robinson and his student James Armit in 1925 and popularized starting in 1959 by the Morrison & Boyd textbook on organic chemistry. The proper use of the symbol is debated: some publications use it to ''any'' cyclic π system, while others use it only for those π systems that obey Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
. Some argue that, in order to stay in line with Robinson's originally intended proposal, the use of the circle symbol should be limited to monocyclic 6 π-electron systems. In this way the circle symbol for a six-center six-electron bond can be compared to the Y symbol for a three-center two-electron bond.
Benzene and derivatives of benzene
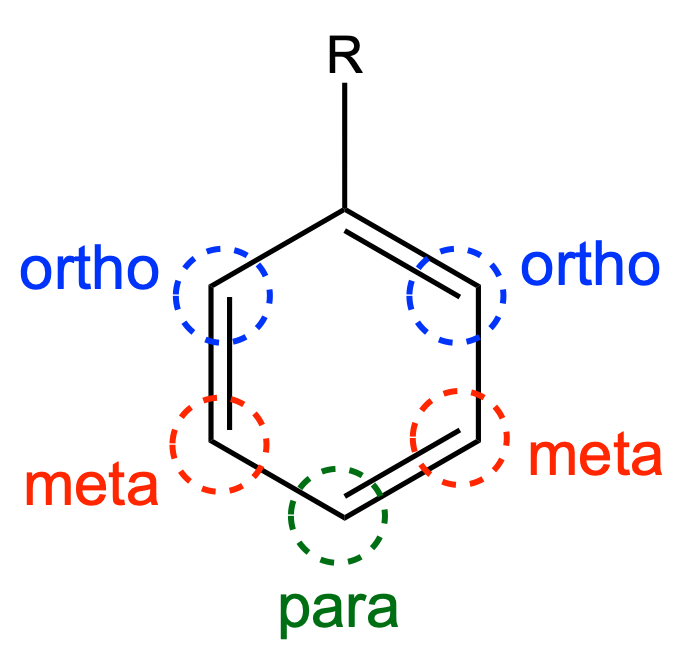
 Benzene derivatives have from one to six
Benzene derivatives have from one to six substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s attached to the central benzene core. Examples of benzene compounds with just one substituent are phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
, which carries a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group, and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
with a methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
group. When there is more than one substituent present on the ring, their spatial relationship becomes important for which the arene substitution patterns ''ortho'', ''meta'', and ''para'' are devised. When reacting to form more complex benzene derivatives, the substituents on a benzene ring can be described as either activated or deactivated, which are electron donating and electron withdrawing respectively. Activators are known as ortho-para directors, and deactivators are known as meta directors. Upon reacting, substituents will be added at the ortho, para or meta positions, depending on the directivity of the current substituents to make more complex benzene derivatives, often with several isomers. Electron flow leading to re-aromatization is key in ensuring the stability of such products.
For example, three isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s exist for cresol
Cresols (also known as hydroxytoluene, toluenol, benzol or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called ''phenolics'') which may be either natural or manufactured. They are also c ...
because the methyl group and the hydroxyl group (both ortho para directors) can be placed next to each other (''ortho''), one position removed from each other (''meta''), or two positions removed from each other (''para''). Given that both the methyl and hydroxyl group are ortho-para directors, the ortho and para isomers are typically favoured. Xylenol
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups at various positions relative to the hydroxyl group. Six isomers exist, of whi ...
has two methyl groups in addition to the hydroxyl group, and, for this structure, 6 isomers exist.
Arene rings can stabilize charges, as seen in, for example, phenol (C6H5–OH), which is acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
at the hydroxyl (OH), as charge on the oxygen (alkoxide –O−) is partially delocalized into the benzene ring.
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
File:Toluol.svg, Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
File:Ethylbenzol.svg, Ethylbenzene
Ethylbenzene is an organic compound with the formula . It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediat ...
File:Cumol.svg, Cumene
File:Para-Xylol - para-xylene.svg, ''p''-Xylene
File:Meta-Xylol - meta-xylene.svg, ''m''-Xylene
File:Ortho-Xylol - ortho-xylene.svg, ''o''-Xylene
File:Mesitylen.svg, Mesitylene
File:1,2,4,5-Tetramethylbenzol.svg, Durene
File:Biphenyl.svg, Biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
File:Phenol.svg, Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
File:Aniline.svg, Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
File:Benzaldehyde.svg, Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
File:Benzoic acid.svg, Benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
File:Benzamide.svg, Benzamide
Benzamide is an organic compound with the chemical formula of C7H7NO. It is the simplest amide derivative of benzoic acid. In powdered form, it appears as a white solid, while in crystalline form, it appears as colourless crystals. It is slightly ...
File:Acetophenone structure.svg, Acetophenone
Non-benzylic arenes
Although benzylic arenes are common, non-benzylic compounds are also exceedingly important. Any compound containing a cyclic portion that conforms toHückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
and is not a benzene derivative can be considered a non-benzylic aromatic compound.
Monocyclic arenes
Ofannulene
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds (' mancude'). They have the general formula C''n''H''n'' (when ''n'' is an even number) or C''n''H''n''+1 (when ''n'' is an odd num ...
s larger than benzene, 2nnulene and 4nnulene are weakly aromatic compounds and 8nnulene, Cyclooctadecanonaene, is aromatic, though strain within the structure causes a slight deviation from the precisely planar structure necessary for aromatic categorization. Another example of a non-benzylic monocyclic arene is the cyclopropenyl (cyclopropenium cation), which satisfies Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
with an n equal to 0. Note, only the cationic form of this cyclic propenyl is aromatic, given that neutrality in this compound would violate either the octet rule or Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
.
Other non-benzylic monocyclic arenes include the aforementioned heteroarenes that can replace carbon atoms with other heteroatoms such as N, O or S. Common examples of these are the five-membered pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
and six-membered pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
, both of which have a substituted nitrogen
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons, also known as polynuclear aromatic compounds (PAHs) are aromatic hydrocarbons that consist of fusedaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
rings and do not contain heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
s or carry substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s. Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
is the simplest example of a PAH. PAHs occur in oil, coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
, and tar deposits, and are produced as byproducts of fuel burning (whether fossil fuel or biomass)."Polycyclic Aromatic Hydrocarbons – Occurrence in foods, dietary exposure and health effects" (PDF). European Commission, Scientific Committee on Food. December 4, 2002. Archived (PDF) from the original on 2022-10-09. As pollutants, they are of concern because some compounds have been identified as carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
ic, mutagen
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in ...
ic, and teratogenic. PAHs are also found in cooked foods. Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures such as grilling or barbecuing, and in smoked fish. They are also a good candidate molecule to act as a basis for the earliest forms of life. In graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
the PAH motif is extended to large 2D sheets.
Reactions
Aromatic ring systems participate in many organic reactions.Substitution
In aromatic substitution, onesubstituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
on the arene ring, usually hydrogen, is replaced by another reagent. The two main types are electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
, when the active reagent is an electrophile, and nucleophilic aromatic substitution, when the reagent is a nucleophile. In radical-nucleophilic aromatic substitution, the active reagent is a radical.
An example of electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
is the nitration of salicylic acid
Salicylic acid is an organic compound with the formula HOC6H4COOH. A colorless (or white), bitter-tasting solid, it is a precursor to and a active metabolite, metabolite of acetylsalicylic acid (aspirin). It is a plant hormone, and has been lis ...
, where a nitro group is added para to the hydroxide substituent:
:leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
, such as a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
, on an aromatic ring
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The e ...
. Aromatic rings usually nucleophilic, but in the presence of electron-withdrawing groups aromatic compounds undergo nucleophilic substitution. Mechanistically, this reaction differs from a common SN2 reaction, because it occurs at a trigonal carbon atom (sp2 hybridization).
Hydrogenation
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of arenes create saturated rings. The compound 1-naphthol
1-Naphthol, or α-naphthol, is an organic compound with the formula . It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene ...
is completely reduced to a mixture of decalin-ol isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s.
:
The compound resorcinol
Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or ''meta- (chemistry), meta''-isomer). Resorcinol crystallizes from benzene as co ...
, hydrogenated with Raney nickel
Raney nickel , also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable s ...
in presence of aqueous sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
forms an enolate which is alkylated with methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
to 2-methyl-1,3-cyclohexandione:
:
Dearomatization
In dearomatization reactions the aromaticity of the reactant is lost. In this regard, the dearomatization is related to hydrogenation. A classic approach isBirch reduction
The Birch reduction or Metal-Ammonia reduction is an organic reaction that is used to convert arenes to Cyclohexa-1,4-diene, 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch (organic chemist), Arthur Birch and i ...
. The methodology is used in synthesis.

Arene-arene interactions
Arene-arene interactions have attracted much attention. Pi-stacking (also called π–π stacking) refers to the presumptively attractive, noncovalentpi interaction
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system ...
s between the pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
s of aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
rings, because of orbital overlap. According to some authors direct stacking of aromatic rings (the "sandwich interaction") is electrostatic
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), mean ...
ally repulsive.
More commonly observed are either a staggered stacking (parallel displaced) or pi-teeing (perpendicular T-shaped) interaction both of which are electrostatic attractive For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare.
Pi stacking is repulsive as it places carbon atoms with partial negative charges from one ring on top of other partial negatively charged carbon atoms from the second ring and hydrogen atoms with partial positive charges on top of other hydrogen atoms that likewise carry partial positive charges. In staggered stacking, one of the two aromatic rings is offset sideways so that the carbon atoms with partial negative charge in the first ring are placed above hydrogen atoms with partial positive charge in the second ring so that the electrostatic interactions become attractive. Likewise, pi-teeing interactions in which the two rings are oriented perpendicular to either other is electrostatically attractive as it places partial positively charged hydrogen atoms in close proximity to partially negatively charged carbon atoms. An alternative explanation for the preference for staggered stacking is due to the balance between van der Waals interactions (attractive dispersion plus Pauli repulsion).
These staggered stacking and π-teeing interactions between aromatic rings are important in nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
stacking within DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
molecules, protein folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, t ...
, template-directed synthesis, materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
, and molecular recognition
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
. Despite the wide use of term pi stacking in the scientific literature, there is no theoretical justification for its use.
Benzene dimer
 The
The benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
dimer is the prototypical system for the study of pi stacking, and is experimentally bound by 8–12 kJ/mol (2–3 kcal/mol) in the gas phase with a separation of 4.96 Å between the centers of mass for the T-shaped dimer. X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
reveals perpendicular and offset parallel configurations for many simple aromatic compounds. Similar offset parallel or perpendicular geometries were observed in a survey of high-resolution x-ray protein crystal structures in the Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules such as proteins and nucleic acids, which is overseen by the Worldwide Protein Data Bank (wwPDB). This structural data is obtained a ...
. Analysis of the aromatic amino acids phenylalanine, tyrosine, histidine, and tryptophan indicates that dimers of these side chains have many stabilizing interactions at distances larger than the average van der Waals radii.
 The relative binding energies of the three geometries of the benzene dimer can be explained by a balance of quadrupole/quadrupole and
The relative binding energies of the three geometries of the benzene dimer can be explained by a balance of quadrupole/quadrupole and London dispersion force
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between at ...
s. While benzene does not have a dipole moment, it has a strong quadrupole moment. The local C–H dipole means that there is positive charge on the atoms in the ring and a correspondingly negative charge representing an electron cloud above and below the ring. The quadrupole moment is reversed for hexafluorobenzene due to the electronegativity of fluorine. The benzene dimer in the sandwich configuration is stabilized by London dispersion forces but destabilized by repulsive quadrupole/quadrupole interactions. By offsetting one of the benzene rings, the parallel displaced configuration reduces these repulsive interactions and is stabilized. The large polarizability of aromatic rings lead to dispersive interactions as major contribution to stacking effects. These play a major role for interactions of nucleobases e.g. in DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
. The T-shaped configuration enjoys favorable quadrupole/quadrupole interactions, as the positive quadrupole of one benzene ring interacts with the negative quadrupole of the other. The benzene rings are furthest apart in this configuration, so the favorable quadrupole/quadrupole interactions evidently compensate for diminished dispersion forces.
According to one model, electron-withdrawing substituents lowers the negative quadrupole of the aromatic ring and thereby favor parallel displaced and sandwich conformations. By contrast, electron donating groups increase the negative quadrupole, which may stabilize a T-shaped configuration with the proper geometry. They used a simple mathematical model based on sigma and pi atomic charges, relative orientations, and van der Waals interactions to qualitatively determine that electrostatics
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles after triboelectric e ...
are dominant in substituent effects.
 Hunter ''et al.'' applied a more sophisticated chemical double mutant cycle with a hydrogen-bonded "zipper" to the issue of substituent effects in pi stacking interactions in proteins. However, the authors note that direct interactions with the ring substituents, discussed below, also make important contributions. Indeed, the interplay of these two factors may result in the complicated substituent- and geometry-dependent behavior of pi stacking interactions.
Some experimental and computational evidence suggests that pi stacking interactions are not governed primarily by electrostatic effects..
The relative contributions pi stacking have been borne out by computation. Trends based on electron donating or withdrawing substituents can be explained by exchange-repulsion and dispersion terms.
A molecular torsion balance from an aryl ester with two conformational states. The folded state had a well-defined pi stacking interaction with a T-shaped geometry, whereas the unfolded state had no aryl–aryl interactions. The NMR chemical shifts of the two conformations were distinct and could be used to determine the ratio of the two states, which was interpreted as a measure of intramolecular forces. The authors report that a preference for the folded state is not unique to aryl esters. For example, the cyclohexyl ester favored the folded state more so than the phenyl ester, and the tert-butyl ester favored the folded state by a preference greater than that shown by any aryl ester. This suggests that aromaticity is not a strict requirement for favorable interaction with an aromatic ring.
Other evidence for non-aromatic pi stacking interactions results include critical studies in theoretical chemistry, explaining the underlying mechanisms of empirical observations. Grimme reported that the interaction energies of smaller dimers consisting of one or two rings are very similar for both aromatic and saturated compounds. This finding is of particular relevance to biology, and suggests that the contribution of pi systems to phenomena such as stacked nucleobases may be overestimated. However, it was shown that an increased stabilizing interaction is seen for large aromatic dimers. As previously noted, this interaction energy is highly dependent on geometry. Indeed, large aromatic dimers are only stabilized relative to their saturated counterparts in a sandwich geometry, while their energies are similar in a T-shaped interaction.
Hunter ''et al.'' applied a more sophisticated chemical double mutant cycle with a hydrogen-bonded "zipper" to the issue of substituent effects in pi stacking interactions in proteins. However, the authors note that direct interactions with the ring substituents, discussed below, also make important contributions. Indeed, the interplay of these two factors may result in the complicated substituent- and geometry-dependent behavior of pi stacking interactions.
Some experimental and computational evidence suggests that pi stacking interactions are not governed primarily by electrostatic effects..
The relative contributions pi stacking have been borne out by computation. Trends based on electron donating or withdrawing substituents can be explained by exchange-repulsion and dispersion terms.
A molecular torsion balance from an aryl ester with two conformational states. The folded state had a well-defined pi stacking interaction with a T-shaped geometry, whereas the unfolded state had no aryl–aryl interactions. The NMR chemical shifts of the two conformations were distinct and could be used to determine the ratio of the two states, which was interpreted as a measure of intramolecular forces. The authors report that a preference for the folded state is not unique to aryl esters. For example, the cyclohexyl ester favored the folded state more so than the phenyl ester, and the tert-butyl ester favored the folded state by a preference greater than that shown by any aryl ester. This suggests that aromaticity is not a strict requirement for favorable interaction with an aromatic ring.
Other evidence for non-aromatic pi stacking interactions results include critical studies in theoretical chemistry, explaining the underlying mechanisms of empirical observations. Grimme reported that the interaction energies of smaller dimers consisting of one or two rings are very similar for both aromatic and saturated compounds. This finding is of particular relevance to biology, and suggests that the contribution of pi systems to phenomena such as stacked nucleobases may be overestimated. However, it was shown that an increased stabilizing interaction is seen for large aromatic dimers. As previously noted, this interaction energy is highly dependent on geometry. Indeed, large aromatic dimers are only stabilized relative to their saturated counterparts in a sandwich geometry, while their energies are similar in a T-shaped interaction.
 A more direct approach to modeling the role of aromaticity was taken by Bloom and Wheeler. The authors compared the interactions between benzene and either 2-methylnaphthalene or its non-aromatic isomer, 2-methylene-2,3-dihydronaphthalene. The latter compound provides a means of conserving the number of p-electrons while, however, removing the effects of delocalization. Surprisingly, the interaction energies with benzene are higher for the non-aromatic compound, suggesting that pi-bond localization is favorable in pi stacking interactions. The authors also considered a homodesmotic dissection of benzene into ethylene and 1,3-butadiene and compared these interactions in a sandwich with benzene. Their calculation indicates that the interaction energy between benzene and homodesmotic benzene is higher than that of a benzene dimer in both sandwich and parallel displaced conformations, again highlighting the favorability of localized pi-bond interactions. These results strongly suggest that aromaticity is not required for pi stacking interactions in this model.
Even in light of this evidence, Grimme concludes that pi stacking does indeed exist. However, he cautions that smaller rings, particularly those in T-shaped conformations, do not behave significantly differently from their saturated counterparts, and that the term should be specified for larger rings in stacked conformations which do seem to exhibit a cooperative pi electron effect.
A more direct approach to modeling the role of aromaticity was taken by Bloom and Wheeler. The authors compared the interactions between benzene and either 2-methylnaphthalene or its non-aromatic isomer, 2-methylene-2,3-dihydronaphthalene. The latter compound provides a means of conserving the number of p-electrons while, however, removing the effects of delocalization. Surprisingly, the interaction energies with benzene are higher for the non-aromatic compound, suggesting that pi-bond localization is favorable in pi stacking interactions. The authors also considered a homodesmotic dissection of benzene into ethylene and 1,3-butadiene and compared these interactions in a sandwich with benzene. Their calculation indicates that the interaction energy between benzene and homodesmotic benzene is higher than that of a benzene dimer in both sandwich and parallel displaced conformations, again highlighting the favorability of localized pi-bond interactions. These results strongly suggest that aromaticity is not required for pi stacking interactions in this model.
Even in light of this evidence, Grimme concludes that pi stacking does indeed exist. However, he cautions that smaller rings, particularly those in T-shaped conformations, do not behave significantly differently from their saturated counterparts, and that the term should be specified for larger rings in stacked conformations which do seem to exhibit a cooperative pi electron effect.
See also
* Aromatic substituents:Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
, Aryloxy
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic co ...
and Arenediyl
* Asphaltene
Asphaltenes are molecular substances that are found in crude oil, along with resins, aromatic hydrocarbons, and saturates (i.e. saturated hydrocarbons such as alkanes). The word "asphaltene" was coined by Jean-Baptiste Boussingault in 1837 whe ...
* Hydrodealkylation
* Simple aromatic rings
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated system, conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found a ...
References
External links
* {{DEFAULTSORT:Aromatic Hydrocarbon