Adhesion (other) on:
[Wikipedia]
[Google]
[Amazon]
 Adhesion is the tendency of dissimilar
Adhesion is the tendency of dissimilar


 In
In 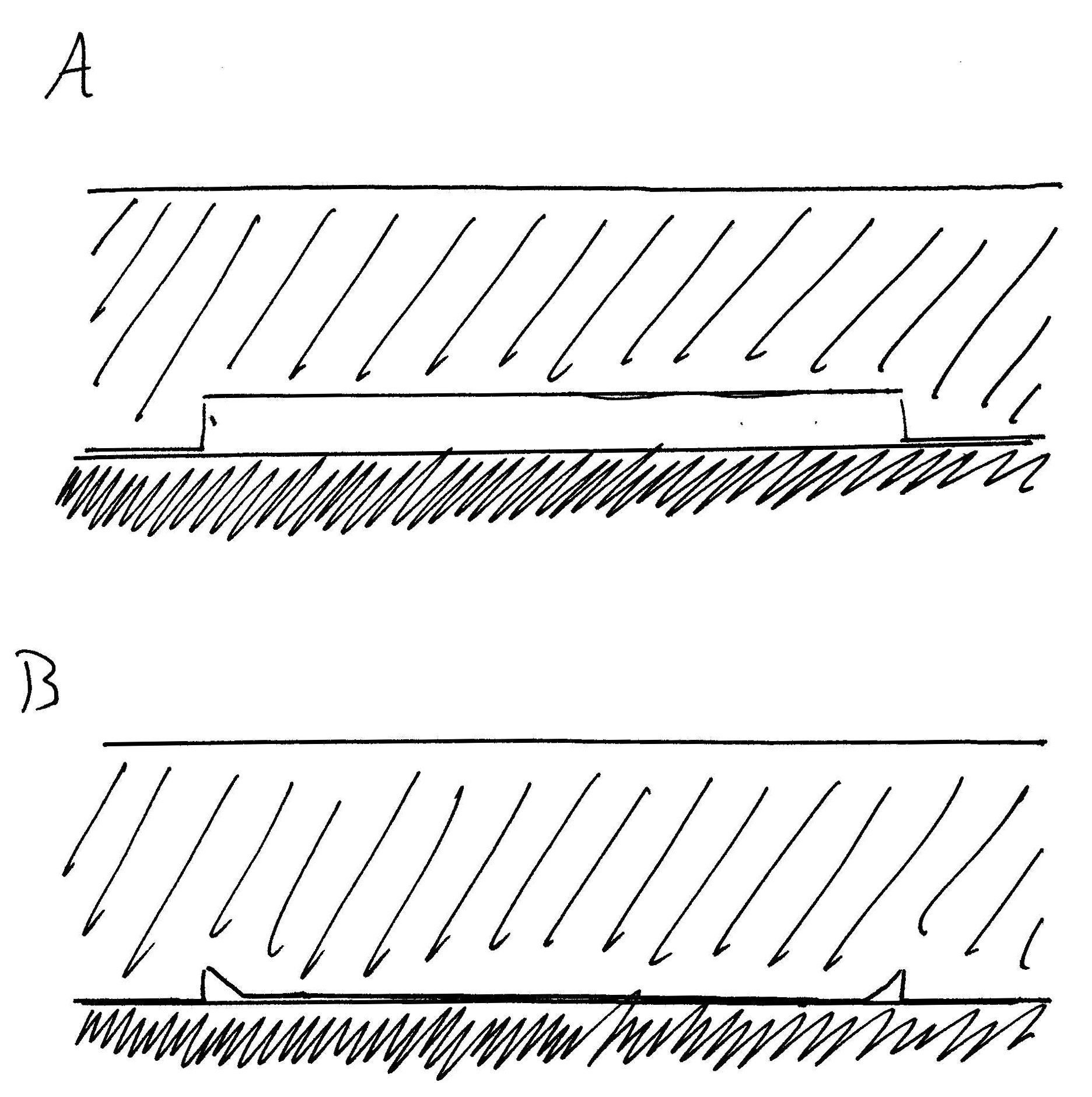 The effect is also apparent in experiments where a
The effect is also apparent in experiments where a
 Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un
Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un
 Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the
Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the
Static Friction at Fractal Interfaces
Tribology International 2016, Volume 93 Technologically advanced adhesive devices sometimes make use of microstructures on surfaces, such as tightly packed periodic posts. These are
 Adhesion is the tendency of dissimilar
Adhesion is the tendency of dissimilar particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
s or surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is ...
s to cling to one another. ( Cohesion refers to the tendency of similar or identical particles and surfaces to cling to one another.)
The force
In physics, a force is an influence that can cause an Physical object, object to change its velocity unless counterbalanced by other forces. In mechanics, force makes ideas like 'pushing' or 'pulling' mathematically precise. Because the Magnitu ...
s that cause adhesion and cohesion can be divided into several types. The intermolecular force
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
s responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion
Adhesion is the tendency of dissimilar particles or interface (matter), surfaces to cling to one another. (Cohesion (chemistry), Cohesion refers to the tendency of similar or identical particles and surfaces to cling to one another.)
The ...
, dispersive adhesion
Dispersive adhesion, also called adsorptive adhesion, is a mechanism for adhesion which attributes attractive forces between two materials to intermolecular interactions between molecules of each material. This mechanism is widely viewed as the ...
, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects.
Surface energy

Surface energy
In surface science, surface energy (also interfacial free energy or surface free energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
is conventionally defined as the work
Work may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
** Manual labour, physical work done by humans
** House work, housework, or homemaking
** Working animal, an ani ...
that is required to build an area of a particular surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is ...
. Another way to view the surface energy is to relate it to the work required to cleave a bulk sample, creating two surfaces. If the new surfaces are identical, the surface energy γ of each surface is equal to half the work of cleavage, W: γ = (1/2)W11.
If the surfaces are unequal, the Young-Dupré equation applies:
W12 = γ1 + γ2 – γ12, where γ1 and γ2 are the surface energies of the two new surfaces, and γ12 is the interfacial energy.
This methodology can also be used to discuss cleavage
Cleavage may refer to:
Science
* Cleavage (crystal), the way in which a crystal or mineral tends to split
* Cleavage (embryo), the division of cells in an early embryo
* Cleavage (geology), foliation of rock perpendicular to stress, a result of ...
that happens in another medium: γ12 = (1/2)W121 = (1/2)W212. These two energy quantities refer to the energy that is needed to cleave one species into two pieces while it is contained in a medium of the other species. Likewise for a three species system: γ13 + γ23 – γ12 = W12 + W33 – W13 – W23 = W132, where W132 is the energy of cleaving species 1 from species 2 in a medium of species 3.
A basic understanding of the terminology of cleavage energy
Cleavage may refer to:
Science
* Cleavage (crystal), the way in which a crystal or mineral tends to split
* Cleavage (embryo), the division of cells in an early embryo
* Cleavage (geology), foliation of rock perpendicular to stress, a result of ...
, surface energy, and surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
is very helpful for understanding the physical state
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma.
Different states are distinguished by the ways the component parti ...
and the events that happen at a given surface, but as discussed below, the theory of these variables also yields some interesting effects that concern the practicality of adhesive surfaces in relation to their surroundings.J. N. Israelachvili, ''Intermolecular and Surface Forces'' (Academic Press, New York, 1985). chap. 15.
Mechanisms
There is no single theory covering adhesion, and particular mechanisms are specific to particular material scenarios. Five mechanisms of adhesion have been proposed to explain why one material sticks to another:Mechanical
Adhesive materials fill thevoids
Void may refer to:
Science, engineering, and technology
* Void (astronomy), the spaces between galaxy filaments that contain no galaxies
* Void (composites), a pore that remains unoccupied in a composite material
* Void, synonym for vacuum, ...
or pores of the surfaces and hold surfaces together by interlocking
In railway signalling, an interlocking is an arrangement of signal apparatus that prevents conflicting movements through an arrangement of tracks such as junctions or crossings. In North America, a set of signalling appliances and tracks inte ...
. Other interlocking phenomena are observed on different length scales. Sewing is an example of two materials forming a large scale mechanical bond, velcro
Velcro IP Holdings LLC, trading as Velcro Companies and commonly referred to as Velcro (pronounced ), is a British privately held company, founded by Swiss electrical engineer George de Mestral in the 1950s. It is the original manufacturer of ho ...
forms one on a medium scale, and some textile adhesives (glue) form one at a small scale.
Chemical
Two materials may form a compound at the joint. The strongest joints are where atoms of the two materials share or swap electrons (known respectively ascovalent bonding
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
or ionic bonding
Ionic bonding is a type of chemical bonding that involves the Coulomb's law, electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in io ...
). A weaker bond is formed if a hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atom in one molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
is attracted to an atom of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, or fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
in another molecule, a phenomenon called hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
.
Chemical adhesion occurs when the surface atoms of two separate surfaces form ionic, covalent, or hydrogen bonds. The engineering principle behind chemical adhesion in this sense is fairly straightforward: if surface molecules can bond, then the surfaces will be bonded together by a network of these bonds. It bears mentioning that these attractive ionic and covalent forces are effective over only very small distances – less than a nanometer
330px, Different lengths as in respect to the Molecule">molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm), or nanometer (American spelling
Despite the va ...
. This means in general not only that surfaces with the potential for chemical bonding need to be brought very close together, but also that these bonds are fairly brittle, since the surfaces then need to be kept close together.
Dispersive
In dispersive adhesion, also known asphysisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely wikt:perturb, perturbed upon adsorption.
Overview
The fundamental interacting force of physisorption is Van der Waals ...
, two materials are held together by van der Waals force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical elec ...
s: the attraction between two molecules, each of which has a region of slight positive and negative charge. In the simple case, such molecules are therefore polar with respect to average charge density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in co ...
, although in larger or more complex molecules, there may be multiple "poles" or regions of greater positive or negative charge. These positive and negative poles may be a permanent property of a molecule (Keesom force
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
s) or a transient effect which can occur in any molecule, as the random movement of electrons within the molecules may result in a temporary concentration of electrons in one region ( London forces).

 In
In surface science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid– gas interfaces, solid– vacuum interfaces, and liquid– gas interfaces. It includes the ...
, the term ''adhesion'' almost always refers to dispersive adhesion. In a typical solid-liquid-gas system (such as a drop of liquid on a solid surrounded by air) the contact angle
The contact angle (symbol ) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interfac ...
is used to evaluate adhesiveness indirectly, while a Centrifugal Adhesion Balance allows for direct quantitative adhesion measurements. Generally, cases where the contact angle is low are considered of higher adhesion per unit area. This approach assumes that the lower contact angle corresponds to a higher surface energy. Theoretically, the more exact relation between contact angle and work of adhesion is more involved and is given by the Young-Dupre equation
Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. These interactions occur in the presence of either a gaseous phase or an ...
. The contact angle of the three-phase system is a function not only of dispersive adhesion (interaction between the molecules in the liquid and the molecules in the solid) but also cohesion (interaction between the liquid molecules themselves). Strong adhesion and weak cohesion results in a high degree of wetting
Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. These interactions occur in the presence of either a gaseous phase or ...
, a lyophilic condition with low measured contact angles. Conversely, weak adhesion and strong cohesion results in lyophobic conditions with high measured contact angles and poor wetting.
London dispersion forces are particularly useful for the function of adhesive devices, because they do not require either surface to have any permanent polarity. They were described in the 1930s by Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German born physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces (London dispersion forces) are to ...
, and have been observed by many researchers. Dispersive forces are a consequence of statistical quantum mechanics. London theorized that attractive forces between molecules that cannot be explained by ionic or covalent interaction can be caused by polar moments within molecules. Multipole
A multipole expansion is a mathematical series representing a function that depends on angles—usually the two angles used in the spherical coordinate system (the polar and azimuthal angles) for three-dimensional Euclidean space, \R^3. Multipol ...
s could account for attraction between molecules having permanent multipole moments that participate in electrostatic interaction
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), meani ...
. However, experimental data showed that many of the compounds observed to experience van der Waals forces had no multipoles at all. London suggested that momentary dipoles are induced purely by virtue of molecules being in proximity to one another. By solving the quantum mechanical system of two electrons as harmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its equilibrium position, experiences a restoring force ''F'' proportional to the displacement ''x'':
\vec F = -k \vec x,
where ''k'' is a positive const ...
s at some finite distance from one another, being displaced about their respective rest positions and interacting with each other's fields, London showed that the energy of this system is given by:
:
While the first term is simply the zero-point energy
Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly Quantum fluctuation, fluctuate in their lowest energy state as described by the Heisen ...
, the negative second term describes an attractive force between neighboring oscillators. The same argument can also be extended to a large number of coupled oscillators, and thus skirts issues that would negate the large scale attractive effects of permanent dipoles cancelling through symmetry, in particular.
The additive nature of the dispersion effect has another useful consequence. Consider a single such dispersive dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
* An electric dipole moment, electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple ...
, referred to as the origin dipole. Since any origin dipole is inherently oriented so as to be attracted to the adjacent dipoles it induces, while the other, more distant dipoles are not correlated with the original dipole by any phase relation (thus on average contributing nothing), there is a net attractive force in a bulk of such particles. When considering identical particles, this is called cohesive force.F. London, "The General Theory of Molecular Forces" (1936).
When discussing adhesion, this theory needs to be converted into terms relating to surfaces. If there is a net attractive energy of cohesion in a bulk of similar molecules, then cleaving this bulk to produce two surfaces will yield surfaces with a dispersive surface energy, since the form of the energy remain the same. This theory provides a basis for the existence of van der Waals forces at the surface, which exist between any molecules having electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
. These forces are easily observed through the spontaneous jumping of smooth surfaces into contact. Smooth surfaces of mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as ''perfect basal cleavage''. Mica is co ...
, gold, various polymers and solid gelatin
Gelatin or gelatine () is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, coll ...
solutions do not stay apart when their separating becomes small enough – on the order of 1–10 nm. The equation describing these attractions was predicted in the 1930s by De Boer and Hamaker:
:
where P is the force (negative for attraction), z is the separation distance, and A is a material-specific constant called the Hamaker constant.
 The effect is also apparent in experiments where a
The effect is also apparent in experiments where a polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling.
PDMS is particularly known for its ...
(PDMS) stamp is made with small periodic post structures. The surface with the posts is placed face down on a smooth surface, such that the surface area in between each post is elevated above the smooth surface, like a roof supported by columns. Because of these attractive dispersive forces between the PDMS and the smooth substrate, the elevated surface – or "roof" – collapses down onto the substrate without any external force aside from the van der Waals attraction. Simple smooth polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
surfaces – without any microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymer ...
s – are commonly used for these dispersive adhesive properties. Decal
A decal (, , ) or transfer is a plastic, cloth, paper, or ceramic substrate that has printed on it a pattern or image that can be moved to another surface upon contact, usually with the aid of heat or water.
The word is short for '' decalc ...
s and stickers that adhere to glass without using any chemical adhesives are fairly common as toys and decorations and useful as removable labels because they do not rapidly lose their adhesive properties, as do sticky tapes that use adhesive chemical compounds.
These forces also act over very small distances – 99% of the work necessary to break van der Waals bonds is done once surfaces are pulled more than a nanometer apart. As a result of this limited motion in both the van der Waals and ionic/covalent bonding situations, practical effectiveness of adhesion due to either or both of these interactions leaves much to be desired. Once a crack is initiated, it propagates easily along the interface because of the brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. ...
nature of the interfacial bonds.
As an additional consequence, increasing surface area often does little to enhance the strength of the adhesion in this situation. This follows from the aforementioned crack failure – the stress at the interface is not uniformly distributed, but rather concentrated at the area of failure.
Electrostatic
Some conducting materials may pass electrons to form a difference inelectrical charge
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
at the joint. This results in a structure similar to a capacitor
In electrical engineering, a capacitor is a device that stores electrical energy by accumulating electric charges on two closely spaced surfaces that are insulated from each other. The capacitor was originally known as the condenser, a term st ...
and creates an attractive electrostatic
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), mean ...
force between the materials.
Diffusive
Some materials may merge at the joint bydiffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
. This may occur when the molecules of both materials are mobile and soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in each other. This would be particularly effective with polymer chains where one end of the molecule diffuses into the other material. It is also the mechanism involved in sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plas ...
. When metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
or ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
powders are pressed together and heated, atoms diffuse from one particle to the next. This joins the particles into one.
 Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un
Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Uncrosslinked
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
(thermoplastics
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
), on the other hand are freer to wander into the adjacent phase by extending tails and loops across the interface.
Another circumstance under which diffusive bonding occurs is "scission". Chain scission is the cutting up of polymer chains, resulting in a higher concentration of distal
Standard anatomical terms of location are used to describe unambiguously the anatomy of humans and other animals. The terms, typically derived from Latin or Greek roots, describe something in its standard anatomical position. This position provi ...
tails. The heightened concentration of these chain ends gives rise to a heightened concentration of polymer tails extending across the interface. Scission is easily achieved by ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
irradiation in the presence of oxygen gas, which suggests that adhesive devices employing diffusive bonding actually benefit from prolonged exposure to heat/light and air. The longer such a device is exposed to these conditions, the more tails are scissed and branch out across the interface.
Once across the interface, the tails and loops form whatever bonds are favorable. In the case of polymer-on-polymer surfaces, this means more van der Waals forces. While these may be brittle, they are quite strong when a large network of these bonds is formed. The outermost layer of each surface plays a crucial role in the adhesive properties of such interfaces, as even a tiny amount of interdigitation – as little as one or two tails of 1.25 angstrom length – can increase the van der Waals bonds by an order of magnitude.
Strength
The strength of the adhesion between two materials depends on which of the above mechanisms occur between the two materials, and the surface area over which the two materials contact. Materials that wet against each other tend to have a larger contact area than those that do not. Wetting depends on the surface energy of the materials. Low surface energy materials such aspolyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
, polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
, polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a corporate spin-of ...
and polyoxymethylene
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. Short-chained POM (chain length ...
are difficult to bond without special surface preparation.
Another factor determining the strength of an adhesive contact is its shape. Adhesive contacts of complex shape begin to detach at the "edges" of the contact area. The process of destruction of adhesive contacts can be seen in the film.
Other effects
In concert with the primary surface forces described above, there are several circumstantial effects in play. While the forces themselves each contribute to the magnitude of the adhesion between the surfaces, the following play a crucial role in the overall strength and reliability of an adhesive device.Stringing
 Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the
Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the stress concentration
In solid mechanics, a stress concentration (also called a stress raiser or a stress riser or notch sensitivity) is a location in an object where the stress (mechanics), stress is significantly greater than the surrounding region. Stress concentra ...
at the point of failure mentioned earlier. Since the stress is now spread out over some area, the stress at any given point has less of a chance of overwhelming the total adhesive force between the surfaces. If failure does occur at an interface containing a viscoelastic
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both Viscosity, viscous and Elasticity (physics), elastic characteristics when undergoing deformation (engineering), deformation. Viscous mate ...
adhesive agent, and a crack does propagate, it happens by a gradual process called "fingering", rather than a rapid, brittle fracture.
Stringing can apply to both the diffusive bonding regime and the chemical bonding regime. The strings of molecules bridging across the gap would either be the molecules that had earlier diffused across the interface or the viscoelastic adhesive, provided that there was a significant volume of it at the interface.
Microstructures
The interplay of molecular scale mechanisms and hierarchical surface structures is known to result in high levels of static friction and bonding between pairs of surfaces.Tribology International 2016, Volume 93
biomimetic
Biomimetics or biomimicry is the emulation of the models, systems, and elements of nature for the purpose of solving complex human problems. The terms "biomimetics" and "biomimicry" are derived from (''bios''), life, and μίμησις ('' mīm ...
technologies inspired by the adhesive abilities of the feet of various arthropod
Arthropods ( ) are invertebrates in the phylum Arthropoda. They possess an arthropod exoskeleton, exoskeleton with a cuticle made of chitin, often Mineralization (biology), mineralised with calcium carbonate, a body with differentiated (Metam ...
s and vertebrate
Vertebrates () are animals with a vertebral column (backbone or spine), and a cranium, or skull. The vertebral column surrounds and protects the spinal cord, while the cranium protects the brain.
The vertebrates make up the subphylum Vertebra ...
s (most notably, gecko
Geckos are small, mostly carnivorous lizards that have a wide distribution, found on every continent except Antarctica. Belonging to the infraorder Gekkota, geckos are found in warm climates. They range from .
Geckos are unique among lizards ...
s). By intermixing periodic breaks into smooth, adhesive surfaces, the interface acquires valuable crack-arresting properties. Because crack initiation requires much greater stress than does crack propagation, surfaces like these are much harder to separate, as a new crack has to be restarted every time the next individual microstructure is reached.
Hysteresis
Hysteresis
Hysteresis is the dependence of the state of a system on its history. For example, a magnet may have more than one possible magnetic moment in a given magnetic field, depending on how the field changed in the past. Plots of a single component of ...
, in this case, refers to the restructuring of the adhesive interface over some period of time, with the result being that the work needed to separate two surfaces is greater than the work that was gained by bringing them together (W > γ1 + γ2). For the most part, this is a phenomenon associated with diffusive bonding. The more time is given for a pair of surfaces exhibiting diffusive bonding to restructure, the more diffusion will occur, the stronger the adhesion will become. The aforementioned reaction of certain polymer-on-polymer surfaces to ultraviolet radiation and oxygen gas is an instance of hysteresis, but it will also happen over time without those factors.
In addition to being able to observe hysteresis by determining if W > γ1 + γ2 is true, one can also find evidence of it by performing "stop-start" measurements. In these experiments, two surfaces slide against one another continuously and occasionally stopped for some measured amount of time. Results from experiments on polymer-on-polymer surfaces show that if the stopping time is short enough, resumption of smooth sliding is easy. If, however, the stopping time exceeds some limit, there is an initial increase of resistance to motion, indicating that the stopping time was sufficient for the surfaces to restructure.
Wettability and absorption
Some atmospheric effects on the functionality of adhesive devices can be characterized by following the theory ofsurface energy
In surface science, surface energy (also interfacial free energy or surface free energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
and interfacial tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to ...
. It is known that γ12 = (1/2)W121 = (1/2)W212. If γ12 is high, then each species finds it favorable to cohere while in contact with a foreign species, rather than dissociate and mix with the other. If this is true, then it follows that when the interfacial tension is high, the force of adhesion is weak, since each species does not find it favorable to bond to the other. The interfacial tension of a liquid and a solid is directly related to the liquid's wettability (relative to the solid), and thus one can extrapolate that cohesion increases in non-wetting liquids and decreases in wetting liquids. One example that verifies this is polydimethyl siloxane rubber, which has a work of self-adhesion of 43.6 mJ/m2 in air, 74 mJ/m2 in water (a nonwetting liquid) and 6 mJ/m2 in methanol (a wetting liquid).
This argument can be extended to the idea that when a surface is in a medium with which binding is favorable, it will be less likely to adhere to another surface, since the medium is taking up the potential sites on the surface that would otherwise be available to adhere to another surface. Naturally this applies very strongly to wetting liquids, but also to gas molecules that could adsorb onto the surface in question, thereby occupying potential adhesion sites. This last point is actually fairly intuitive: Leaving an adhesive exposed to air too long gets it dirty, and its adhesive strength will decrease. This is observed in the experiment: when mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as ''perfect basal cleavage''. Mica is co ...
is cleaved in air, its cleavage energy, W121 or Wmica/air/mica, is smaller than the cleavage energy in vacuum, Wmica/vac/mica, by a factor of 13.
Lateral adhesion
Lateral adhesion is associated with sliding one object on a substrate, such as sliding a drop on a surface. When the two objects are solids, either with or without a liquid between them, the lateral adhesion is described as ''friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. Types of friction include dry, fluid, lubricated, skin, and internal -- an incomplete list. The study of t ...
''. However, the behavior of lateral adhesion between a drop and a surface is tribologically very different from friction between solids, and the naturally adhesive contact between a flat surface and a liquid drop makes the lateral adhesion in this case, an individual field. Lateral adhesion can be measured using the centrifugal adhesion balance (CAB), which uses a combination of centrifugal and gravitational forces to decouple the normal and lateral forces in the problem.
See also
*Adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advantage ...
* Adhesive bonding
Adhesive bonding is a joining technique used in the manufacture and repair of a wide range of products. Along with welding and soldering, adhesive bonding is one of the basic joining processes. In this technique, components are bonded together usi ...
* Bacterial adhesin Bacterial adhesins are cell-surface components or appendages of bacteria that facilitate adhesion or adherence to other cells or to surfaces, usually in the host they are infecting or living in. Adhesins are a type of virulence factor.
Adherence is ...
* Capillary action
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of external forces like Gravitation, gravity.
The effe ...
* Cell adhesion
Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as Cell_junction, cell junc ...
* Contact mechanics
Contact mechanics is the study of the Deformation (mechanics), deformation of solids that touch each other at one or more points. A central distinction in contact mechanics is between Stress (mechanics), stresses acting perpendicular to the cont ...
* Electroadhesion
* Fracture mechanics
Fracture mechanics is the field of mechanics concerned with the study of the propagation of cracks in materials. It uses methods of analytical solid mechanics to calculate the driving force on a crack and those of experimental solid mechanics t ...
* Galling
Galling is a form of wear caused by adhesion between sliding surfaces. When a material galls, some of it is pulled with the contacting surface, especially if there is a large amount of force compressing the surfaces together. Galling is cau ...
* Insect adhesion
* Meniscus
* Mucoadhesion
* Pressure-sensitive adhesive
Pressure-sensitive adhesive (PSA, self-adhesive, self-stick adhesive) is a type of nonreactive adhesive which forms a bond when pressure is applied to bond the adhesive with a surface. No solvent, water, or heat is needed to activate the adhesive ...
* Rail adhesion
An adhesion railway relies on adhesion traction to move the train, and is the most widespread and common type of railway in the world. Adhesion traction is the friction between the drive wheels and the steel rail. Since the vast majority of railw ...
* Synthetic setae
Synthetic setae emulate the setae found on the gecko feet, toes of a gecko and scientific research in this area is driven towards the development of Dry glue, dry adhesives. Geckos have no difficulty mastering vertical walls and are apparently cap ...
* Wetting
Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. These interactions occur in the presence of either a gaseous phase or ...
* Cohesion number
The Cohesion number (''Coh'') is a useful dimensionless number in particle technology by which the cohesivity of different powders can be compared. This is especially useful in DEM simulations ( Discrete Element Method) of granular materials whe ...
References
Further reading
* John Comyn, ''Adhesion Science'', Royal Society of Chemistry Paperbacks, 1997 * A.J. Kinloch, ''Adhesion and Adhesives: Science and Technology'', Chapman and Hall, 1987 {{Authority control Materials science Chemical properties Intermolecular forces Articles containing video clips