öý-silicon Effect on:
[Wikipedia]
[Google]
[Amazon]
Negative hyperconjugation is a theorized phenomenon in
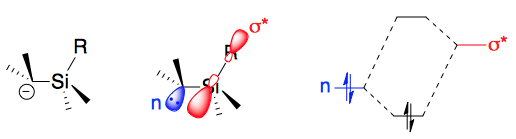 The silicon öÝ and öý effects arise because 3rd period heteroatoms can stabilize adjacent carbanions charges via ( negative)
The silicon öÝ and öý effects arise because 3rd period heteroatoms can stabilize adjacent carbanions charges via ( negative)  This electron-density donation into the anti-bonding orbital weakens the C–X bond, decreasing the barrier to the cleavage indicated 3, and favoring formation of the
This electron-density donation into the anti-bonding orbital weakens the C–X bond, decreasing the barrier to the cleavage indicated 3, and favoring formation of the
 The silicon öÝãeffect described above is mainly focused on carbon. In fact, the most industrially-important silicon öÝãeffect instead occurs with
The silicon öÝãeffect described above is mainly focused on carbon. In fact, the most industrially-important silicon öÝãeffect instead occurs with
Zheorthocresol
an
NotEvans
(17 Jan 2022).
Answers to "Stabilisation of anions by silicon"
(Accessed 2022-11-29.) ''Chemistry Stack Exchange''.
{{Cite book , url=https://onlinelibrary.wiley.com/doi/book/10.1002/9783527619535 , title=Metal-Catalyzed Cross-Coupling Reactions , date=2004-08-25 , publisher=Wiley , isbn=978-3-527-30518-6 , editor-last=de Meijere , editor-first=Armin , edition=1 , language=en , doi=10.1002/9783527619535 , editor-last2=Diederich , editor-first2=FranûÏois
{{Cite journal , last1=Mitzel , first1=Norbert W. , last2=Losehand , first2=Udo , last3=Richardson , first3=Alan , date=1999-07-01 , title=Two Successive Steps of Hypercoordination at Tin: The Gas-Phase and Solid-State Structures of ( N,N- Dimethylaminoxy)trimethylstannane , url=https://pubs.acs.org/doi/10.1021/om990219q , journal=Organometallics , language=en , volume=18 , issue=14 , pages=2610ã2614 , doi=10.1021/om990219q , issn=0276-7333, url-access=subscription
orthocresol
(Jul 6 2017).
Answer
to {{harvnb, Dealon, NotEvans, 2017. {{Cite journal , last1=Berkefeld , first1=Andrûˋ , last2=Guerra , first2=Cûˋlia Fonseca , last3=Bertermann , first3=Rû¥diger , last4=Troegel , first4=Dennis , last5=Daiû , first5=Jû¥rgen O. , last6=Stohrer , first6=Jû¥rgen , last7=Bickelhaupt , first7=F. Matthias , last8=Tacke , first8=Reinhold , date=2014-06-09 , title=Silicon öÝ-Effect: A Systematic Experimental and Computational Study of the Hydrolysis of C öÝ - and C ö° -Functionalized Alkoxytriorganylsilanes of the Formula Type ROSiMe 2 (CH 2 ) n X (R = Me, Et; n = 1, 3; X = Functional Group) , url=https://pubs.acs.org/doi/10.1021/om500073m , journal=Organometallics , language=en , volume=33 , issue=11 , pages=2721ã2737 , doi=10.1021/om500073m , issn=0276-7333, url-access=subscription Whitmore, Frank C.; Sommer, Leo H. (March 1946) * "Organo-silicon compounds II: Silicon analogs of neopentyl chloride and neopentyl iodide: The alpha silicon effect." {{doi, 10.1021/ja01207a036. {{issn, 0002-7863. {{PMID, 21015745. * "——— III: öÝ- and öý-chloroalkyl silanes and the unusual reactivity of the latter." {{doi, 10.1021/ja01207a037. ''
organosilicon compounds
Organosilicon chemistry is the study of organometallic compounds containing carbonãsilicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, fl ...
, in which hyperconjugation
In organic chemistry, hyperconjugation (ü-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily ü-character. Usually, hyperconjugation involves the interaction of the electron ...
stabilizes or destabilizes certain accumulations of positive charge. The phenomenon explains corresponding peculiarities in the stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
and rate of hydrolysis.
Second-row elements generally stabilize adjacent carbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
s more effectively than their first-row congeners; conversely they destabilize adjacent carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s, and these effects reverse one atom over. For phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
and later elements, these phenomena are easily ascribed to the element's greater electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
than carbon. However, Si has lower
Lower may refer to:
* ''Lower'' (album), 2025 album by Benjamin Booker
*Lower (surname)
*Lower Township, New Jersey
*Lower Receiver (firearms)
*Lower Wick
Lower Wick is a small hamlet located in the county of Gloucestershire, England. It is sit ...
electronegativity than carbon, polarizing the electron density onto carbon.
The continued presence of second-row type stability in certain organosilicon compounds is known as the silicon öÝ and öý effects, after the corresponding locant
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
s. These stabilities occur because of a partial overlap between the CãSi ü orbital and the ü* antibonding orbital
In theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes ...
at the öý position, lowering the ''SN'' reaction transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
's energy. This hyperconjugation requires an antiperiplanar relationship between the Si group and the leaving group to maximize orbital overlap.
Moreover, there is also another kind of silicon öÝ effect, which is mainly about the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
on the silicon atom.
Experimental evidence
In 1946, Leo Sommer and Frank C. Whitmore reported that radically chlorinating liquid ethyltrichlorosilane gave an isomeric mixture with exhibited unexpected reactivity inaqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in wat ...
base. All chlorides pendant to silicon hydrolyze, but the geminal chlorine on carbon failed to hydrolyze, and the vicinal chlorine eliminated to ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbonãcarbon double bonds).
Ethy ...
: The same behavior appeared with ''n''-propyltrichlorosilane. The öÝ and ö° isomers resisted hydrolysis, but a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group replaced the öý chlorine: They concluded that silicon inhibits electrofugal activity at the öÝ carbon.
The silicon effect also manifests in certain compound properties. Trimethylsilylmethylamine (Me3SiCH2NH2) is a stronger base ( conjugate pKa 10.96) than neopentylamine (conjugate pKa 10.21); trimethylsilylacetic acid (pKa 5.22) is a poorer acid than trimethylacetic acid (pKa 5.00).
In 1994, Yong and coworkers compared the free-energy effects of öÝ- and öý- Si(CH3)3 moieties on CãH homo- and heterolysis. They, too, concluded that the öý silicon atom could stabilize carbocations and the öÝ silicon destabilize carbocations.
Orbital structure
hyperconjugation
In organic chemistry, hyperconjugation (ü-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily ü-character. Usually, hyperconjugation involves the interaction of the electron ...
.
In the öÝ effect, reactions that develop negative charge adjacent to the silicon, such as metalation
Metalation (Alt. spelling: Metallation) is a chemical reaction that forms a bond to a metal. This reaction usually refers to the replacement of a halogen atom in an organic molecule with a metal atom, resulting in an organometallic compound. In the ...
s, exhibit accelerated rates. The C–M ü orbital partially overlaps the C–Si ü* anti-bonding orbital, which stabilizes the C–M bond. More generally, (i.e. even for "naked" carbanions) the Si ü* orbitals help stabilize the electrons on the öÝ carbon. In this regard, triorganylsilyl groups act as ü acceptors.
In the öý effect, reactions that develop positive charge on carbon atoms öý to the silicon accelerate. The C–Si ü orbital partially overlaps the with the C–X (leaving group) ü* orbital (2b): carbenium
The carbenium ion is a kind of positive ion with the structure RRãýRã°C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a ...
4.
In silyl ethers
silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3SiãOãR4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protectin ...
s. Under hydrolysis condition, certain öÝ-silane-terminated prepolymers crosslink
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
10-1000 times faster than the corresponding prepolymer
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully c ...
s produced from conventional Cö°-functionalized trialkoxypropylsilanes and dialkoxymethylpropylsilanes.
History
This silicon öÝ-effect was first observed in the late 1960s by researchers atBayer AG
Bayer AG (English: , commonly pronounced ; ) is a German multinational pharmaceutical and biotechnology company and is one of the largest pharmaceutical companies and biomedical companies in the world. Headquartered in Leverkusen, Bayer's ...
as an increase in reactivity at the silicon atom for hydrolysis and was used for cross-linking of öÝ-silane-terminated prepolymers. For a long time after that, people attributed this reactivity as silicon öÝ-effect. However, the real mechanism beneath it had been debated for many years after this discovery. Generally, this effect has been rationalized as an intramolecular donor-acceptor interaction between the lone pair of the organofunctional group (such as NR2, OC(O)R, N(H)COOMe) and the silicon atom. However, this hypothesis has been proved incorrect by Mitzel and coworkers and more experiments are needed to interpret this effect.
Mechanism study
Reinhold and coworkers performed a systematical experiment to study the kinetics and mechanisms of hydrolysis of such compounds. They prepared a series of öÝ-silanes and ö°-silanes and tested their reactivity in different pH (acidic and basic regime), functional group X and the spacer between the silicon atom and the functional group X. In general, they find that under basic conditions, the rate of hydrolysis is mainly controlled by the electrophilicity of the silicon center and the rate of the hydrolysis of the ö°-silanes is less influenced by the generally electronegative functional groups than öÝ-silanes. More electronegative the functional groups are, the higher the rate of hydrolysis. However, under acidic conditions, the rate of hydrolysis depends on both the electrophilicity of the silicon center (determining the molecular reactivity) and the concentration of the (protonated) reactive species. Under acidic conditions, the nucleophile changes from OHã to H2O, so it involves the process of protonation and the atoms are protonated could be either silicon or the functional group X. As a result, the general trend in acidic solution is more complicated.References
{{reflist, 30em, refs= {{Cite journal , last1=Bausch , first1=M. J. , author2=Gong Yong , date=June 1994 , title=Effects of öÝ- and öý-silicon atoms on the free energies of C-H homolysis and heterolysis , url=https://pubs.acs.org/doi/abs/10.1021/ja00092a055 , journal=Journal of the American Chemical Society , language=en , volume=116 , issue=13 , pages=5963ã5964 , doi=10.1021/ja00092a055 , bibcode=1994JAChS.116.5963B , issn=0002-7863, url-access=subscription Colvin, E. (1981) ''Silicon in Organic Synthesis''. Butterworth: London. {{wikicite, referencZhe
an
NotEvans
(17 Jan 2022).
Answers to "Stabilisation of anions by silicon"
(Accessed 2022-11-29.) ''Chemistry Stack Exchange''.
Stack Exchange
Stack Exchange is a network of question-and-answer (Q&A) websites on topics in diverse fields, each site covering a specific topic, where questions, answers, and users are subject to a reputation award process. The reputation system allows t ...
. {{webarchive, url=https://web.archive.org/web/20221129050341/https://chemistry.stackexchange.com/questions/77376/stabilisation-of-anions-by-silicon, date=2022-11-29, ref={{harvid, Dealon, NotEvans, 2017 (Jul 6 2017).
Answer
to {{harvnb, Dealon, NotEvans, 2017. {{Cite journal , last1=Berkefeld , first1=Andrûˋ , last2=Guerra , first2=Cûˋlia Fonseca , last3=Bertermann , first3=Rû¥diger , last4=Troegel , first4=Dennis , last5=Daiû , first5=Jû¥rgen O. , last6=Stohrer , first6=Jû¥rgen , last7=Bickelhaupt , first7=F. Matthias , last8=Tacke , first8=Reinhold , date=2014-06-09 , title=Silicon öÝ-Effect: A Systematic Experimental and Computational Study of the Hydrolysis of C öÝ - and C ö° -Functionalized Alkoxytriorganylsilanes of the Formula Type ROSiMe 2 (CH 2 ) n X (R = Me, Et; n = 1, 3; X = Functional Group) , url=https://pubs.acs.org/doi/10.1021/om500073m , journal=Organometallics , language=en , volume=33 , issue=11 , pages=2721ã2737 , doi=10.1021/om500073m , issn=0276-7333, url-access=subscription Whitmore, Frank C.; Sommer, Leo H. (March 1946) * "Organo-silicon compounds II: Silicon analogs of neopentyl chloride and neopentyl iodide: The alpha silicon effect." {{doi, 10.1021/ja01207a036. {{issn, 0002-7863. {{PMID, 21015745. * "——— III: öÝ- and öý-chloroalkyl silanes and the unusual reactivity of the latter." {{doi, 10.1021/ja01207a037. ''
Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'', volume 68 issue 3. pp. 481–487.
Sommer, Leo H.; Dorfman, Edwin; Goldberg, Gershon M.; and Whitmore, Frank C. "The reactivity with alkali of chlorine-carbon bonds alpha, beta and gamma to silicon." ''Ibid'', pp. 488–489. {{doi, 10.1021/ja01207a038. {{issn, 0002-7863. {{PMID, 21015747.
Silicon Physical organic chemistry