Î -interactions on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal (cationic or neutral), an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.
 * Aromatic–aromatic interactions (π stacking): involves interactions of aromatic molecules with each other.
**Arene–perfluoroarene interaction: electron-rich benzene ring interacts with electron-poor
* Aromatic–aromatic interactions (π stacking): involves interactions of aromatic molecules with each other.
**Arene–perfluoroarene interaction: electron-rich benzene ring interacts with electron-poor  *π donor–acceptor interactions: interaction between low energy empty orbital (acceptor) and a high-energy filled orbital (donor).
*π donor–acceptor interactions: interaction between low energy empty orbital (acceptor) and a high-energy filled orbital (donor).
 *Anion–π interactions: interaction of anion with π system
* Cation–π interactions: interaction of a cation with a π system
*C–H–π interactions: interaction of C-H with π system: These interactions are well studied using experimental as well as computational techniques.
*Anion–π interactions: interaction of anion with π system
* Cation–π interactions: interaction of a cation with a π system
*C–H–π interactions: interaction of C-H with π system: These interactions are well studied using experimental as well as computational techniques.
Buckycatcher JACS 2007 V129 p3843.jpg, A

\pi - \pi , CH-\pi and \pi -cation interactions are widely observed motifs in supramolecular assembly and \pi-\pi concerns the direct interactions between two -systems; and cation-\pi interaction arises from the electrostatic interaction of a cation with the face of the -system. Unlike these two interactions, the CH-\pi interaction arises mainly from charge transfer between the C–H orbital and the -system.
Ď€ systems contribute to
 systems are important building blocks in
systems are important building blocks in \pi - \pi , CH-\pi and \pi -cation interactions are widely used in supramolecular assembly and \pi-\pi concerns the direct interactions between two -systems; and cation-\pi interaction arises from the electrostatic interaction of a cation with the face of the -system. Unlike these two interactions, the CH-\pi interaction arises mainly from charge transfer between the C–H orbital and the -system.
Larry Wolf (2011): π-π (π-Stacking) interactions: origin and modulation
Types
The most common types of π-interactions involve: *Metal–π interactions: involves interaction of a metal and the face of a π system, the metal can be a cation (known as cation–π interactions) or neutral *Polar–π interactions: involves interaction of a polar molecule and quadrupole moment a π system.hexafluorobenzene
Hexafluorobenzene, HFB or perfluorobenzene is an organofluorine compound with the chemical formula . In this derivative of benzene, all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although i ...
.
Examples
fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
bound in a buckycatcher through aromatic stacking interactions.
File:24 fig. 2.png, The Stoddart synthesis of catenane...
File:QUATERNARY LIGAND BINDING TO AROMATIC RESIDUES IN THE ACTIVE-SITE GORGE OF ACETYLCHOLINESTERASE.png, Tacrine
Tacrine is a centrally acting acetylcholinesterase inhibitor and indirect cholinergic agonist (parasympathomimetic). It was the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer's disease, and was marketed u ...
bound to acetylcholinesterase (PDB 1ACJ). A pi stacking interaction between tacrine (blue) and Trp84 (red) is proposed
Polar-pi.svg, Polar π interaction between water molecule and benzene
Areneperfluoroarene.svg, Arene perfluoroarene stacking
SegStackEdgeOnHMTFCQ.jpg, Edge-on view of portion of crystal structure of hexamethylene TTF/TCNQ charge transfer salt, highlighting the segregated stacking.
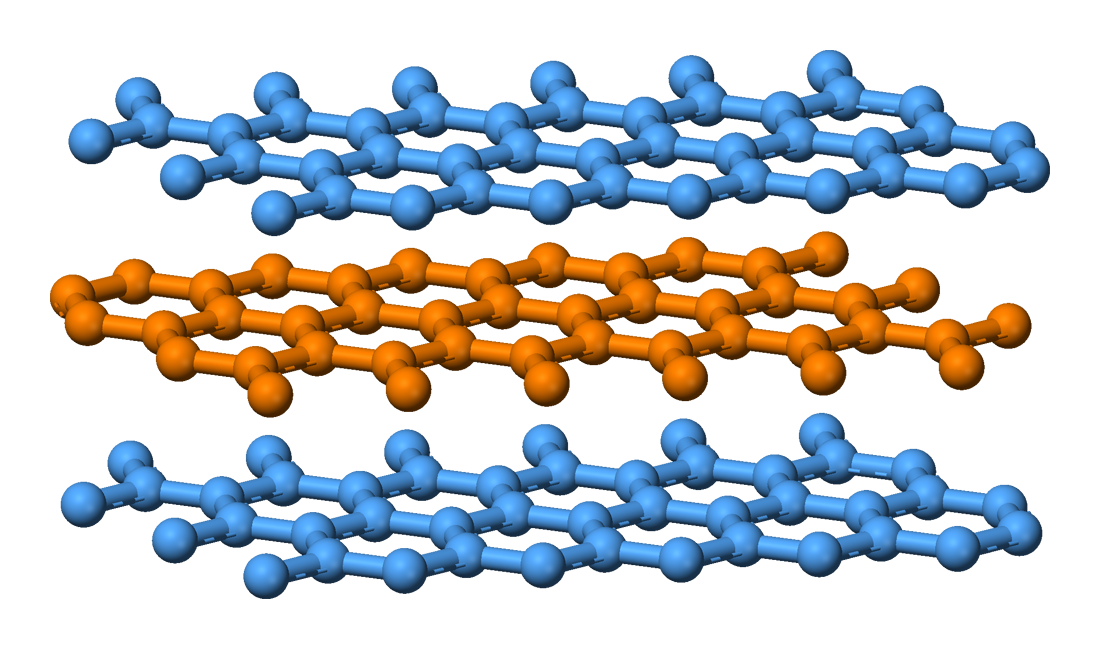
Graphite

Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
consists of stacked sheets of covalently bonded carbon. The individual layers are called graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
. In each layer, each carbon atom is bonded to three other atoms forming a continuous layer of sp2 bonded carbon hexagons, like a honeycomb lattice
The hexagonal lattice (sometimes called triangular lattice) is one of the five two-dimensional Bravais lattice types. The symmetry category of the lattice is wallpaper group p6m. The primitive translation vectors of the hexagonal lattice form an ...
with a bond length of 0.142 nm, and the distance between planes is 0.335 nm.
Ď€-effects in biological systems
Cation-Ď€ interactions are important for theacetylcholine
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Par ...
(Ach) neurotransmitter. The structure of acetylcholine esterase
Acetylcholinesterase ( HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of ac ...
includes 14 highly conserved aromatic residues. The trimethyl ammonium group of Ach binds to the aromatic residue of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
(Trp). The indole site provides a much more intense region of negative electrostatic potential than benzene and phenol residue of Phe and Tyr.
Pi–pi and cation–pi interactions are important in rational drug design. One example is the FDA-approved acetylcholinesterase
Acetylcholinesterase (HUGO Gene Nomenclature Committee, HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme th ...
(AChE) inhibitor tacrine
Tacrine is a centrally acting acetylcholinesterase inhibitor and indirect cholinergic agonist (parasympathomimetic). It was the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer's disease, and was marketed u ...
which is used in the treatment of Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
. Tacrine is proposed to have a pi stacking interaction with the indolic ring of Trp84, and this interaction has been exploited in the rational design of novel AChE inhibitors.Supramolecular assembly

recognition
Recognition may refer to:
Machine learning
*Pattern recognition, a branch of machine learning which encompasses the meanings below
Biometric
* Recognition of human individuals, or biometrics, used as a form of identification and access control
...
.
supramolecular assembly
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
. Some catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be se ...
s feature π–π interactions. The major challenge for the synthesis of catenane is to interlock molecules in a controlled fashion. Stoddart and co-workers developed a series of systems utilizing the strong π–π interactions between electron-rich benzene derivatives and electron-poor pyridinium rings. atanene was synthesized by reacting bis(pyridinium) (A), bisparaphenylene-34-crown-10 (B), and 1, 4-bis(bromomethyl)benzene (C) (Fig. 2). The π–π interaction between A and B directed the formation of an interlocked template intermediate that was further cyclized by substitution reaction with compound C to generate the atenane product.
Charge transfer salts
A combination oftetracyanoquinodimethane
Tetracyanoquinodimethane (TCNQ) is an organic compound with the chemical formula . It is an orange crystalline solid. This cyanocarbon, a relative of para-quinone, is an electron acceptor that is used to prepare charge transfer salts, which ar ...
(TCNQ) and tetrathiafulvalene
Tetrathiafulvalene (TTF) is an organosulfur compound with the formula . It is the parent of many tetrathiafulvenes. Studies on these heterocyclic compound contributed to the development of molecular electronics, although no practical applications ...
(TTF) forms a strong charge-transfer complex referred to as ''TTF-TCNQ''. The solid shows almost metallic electrical conductance. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks.
Anion–π interactions
Anion and π–aromatic systems (typically electron-deficient) create an interaction that is associated with the repulsive forces of the structures. These repulsive forces involve electrostatic and anion-induced polarized interactions. This force allows for the systems to be used as receptors and channels in supramolecular chemistry for applications in the medical (synthetic membranes, ion channels) and environmental fields (e.g. sensing, removal of ions from water). The first X-ray crystal structure that depicted anion–π interactions was reported in 2004. In addition to this being depicted in the solid state, there is also evidence that the interaction is present in solution.π-effects in biological systems
Ď€-effects have an important contribution to biological systems since they provide a significant amount of binding enthalpy. Neurotransmitters produce most of their biological effect by binding to the active site of a protein receptor. Xation-Ď€ interactions are important for theacetylcholine
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Par ...
(Ach) neurotransmitter. The structure of acetylcholine esterase
Acetylcholinesterase ( HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of ac ...
includes 14 highly conserved aromatic residues. The trimethyl ammonium group of Ach binds to the aromatic residue of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
(Trp). The indole site provides a much more intense region of negative electrostatic potential than benzene and phenol residue of Phe and Tyr.
In supramolecular assembly
 systems are important building blocks in
systems are important building blocks in supramolecular assembly
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
because of their versatile noncovalent interactions with various functional groups. Particularly, recognition
Recognition may refer to:
Machine learning
*Pattern recognition, a branch of machine learning which encompasses the meanings below
Biometric
* Recognition of human individuals, or biometrics, used as a form of identification and access control
...
.
See also
* Noncovalent interaction *Dispersion (chemistry)
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
Dispersions are classified in a number of diff ...
* Cation–pi interaction
* Intercalation (biochemistry)
In biochemistry, intercalation is the insertion of molecules between the planar bases of deoxyribonucleic acid (DNA). This process is used as a method for analyzing DNA and it is also the basis of certain kinds of poisoning.
There are several ...
* Intercalation (chemistry)
Intercalation is the reversible inclusion or insertion of a molecule (or ion) into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides.
:
Examples Graphite
One famous intercalation hos ...
External links
*Larry Wolf (2011): π-π (π-Stacking) interactions: origin and modulation
References
{{Chemical bonds Intermolecular forces Organic chemistry Chemical bonding Supramolecular chemistry