|
Zircaloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption Nuclear cross section, cross-section of thermal neutrons, high hardness, ductility and corrosion resistance. One of the main uses of zirconium alloys is in nuclear technology, as Cladding (nuclear fuel), cladding of fuel rods in nuclear reactors, especially light water reactor, water reactors. A typical composition of nuclear-grade zirconium alloys is more than 95 Mass fraction (chemistry), weight percent zirconium and less than 2% of tin, niobium, iron, chromium, nickel and other metals, which are added to improve mechanical properties and corrosion resistance. The water cooling of reactor zirconium alloys elevates requirement for their resistance to oxidation-related nodular corrosion. Furthermore, oxidative reaction of zirconium with water releases hydrogen gas, which partly diffuses into the alloy and forms zirconium hydrides. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium Hydride
Zirconium hydride describes an alloy made by combining zirconium and hydrogen. Hydrogen acts as a hardening agent, preventing dislocations in the zirconium atom crystal lattice from sliding past one another. Varying the amount of hydrogen and the form of its presence in the zirconium hydride (precipitated phase) controls qualities such as the hardness (materials science), hardness, ductility, and ultimate tensile strength, tensile strength of the resulting zirconium hydride. Zirconium hydride with increased hydrogen content can be made harder and stronger than zirconium, but such zirconium hydride is also less ductile than zirconium. Material properties Zirconium is found in the Earth's crust (geology), crust only in the form of an ore, usually a zirconium silicate, such as zircon. Zirconium is extracted from zirconium ore by removing the oxygen and silica. This process, known as the Kroll process, was first applied to titanium. The Kroll process results in an alloy containing ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Rod
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other nuclear devices to generate energy. Oxide fuel For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state. Uranium dioxide Uranium dioxide is a black semiconducting solid. It can be made by heating uranyl nitrate to form . : This is then converted by heating with hydrogen to form UO2. It can be made from enriched uranium hexafluoride by reacting with ammonia to form a solid called ammonium diuranate, . This is then heated ( calcined) to form and U3O8 which is then converted by heating with hydrogen or ammonia to form UO2. The UO2 is mixed with an organic binder and pressed into pellets. The pellets are then fired at a much highe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, Ductility, ductile, malleable and corrosion-resistant. The name ''zirconium'' is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian Language, Persian ''Jargoon, zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin. Zirconium forms a variety of inorganic chemistry, inorganic compounds, such as zirconium dioxide, and organometallic compounds, such as zirconocene dichloride. Five isotope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CANDU Reactor
The CANDU (CANada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide (heavy water) moderator and its use of (originally, natural) uranium fuel. CANDU reactors were first developed in the late 1950s and 1960s by a partnership between Atomic Energy of Canada Limited (AECL), the Hydro-Electric Power Commission of Ontario, Canadian General Electric, and other companies. There have been two major types of CANDU reactors, the original design of around 500 MWe that was intended to be used in multi-reactor installations in large plants, and the optimized CANDU 6 in the 600 MWe class that is designed to be used in single stand-alone units or in small multi-unit plants. CANDU 6 units were built in Quebec and New Brunswick, as well as Pakistan, Argentina, South Korea, Romania, and China. A single example of a non-CANDU 6 design was sold to India. The multi-unit design was used onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons production and Research reactor, research. Fissile material, Fissile nuclei (primarily uranium-235 or plutonium-239) absorb single neutron, neutrons and split, releasing energy and multiple neutrons, which can induce further fission. Reactors stabilize this, regulating Neutron absorber, neutron absorbers and neutron moderator, moderators in the core. Fuel efficiency is exceptionally high; Enriched uranium#Low-enriched uranium (LEU), low-enriched uranium is 120,000 times more energy dense than coal. Heat from nuclear fission is passed to a working fluid Nuclear reactor#By coolant, coolant. In commercial reactors, this drives Turbine, turbines and electrical generator shafts. Some reactors are used for district heating, and isotopes, isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cladding (nuclear Fuel)
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other nuclear devices to generate energy. Oxide fuel For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state. Uranium dioxide Uranium dioxide is a black semiconducting solid. It can be made by heating uranyl nitrate to form . : This is then converted by heating with hydrogen to form UO2. It can be made from enriched uranium hexafluoride by reacting with ammonia to form a solid called ammonium diuranate, . This is then heated (calcined) to form and U3O8 which is then converted by heating with hydrogen or ammonia to form UO2. The UO2 is mixed with an organic binder and pressed into pellets. The pellets are then fired at a much higher temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can Permeation, permeate solid metals. Once absorbed, hydrogen lowers the Stress (mechanics), stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs in steels, as well as in iron, nickel, titanium, cobalt, and their alloys. Copper, aluminium, and stainless steels are less susceptible to hydrogen embrittlement. The essential facts about the nature of hydrogen embrittlement have been known since the 19th century. Hydrogen embrittlement is maximised at around room temperature in steels, and most metals are relatively immune to hydrogen embrittlement at temperatures above 150 °C. Hydrogen embrittlement requires the presence of both atomic ("diffusible") hydrogen and a Stress (mechanics), mechanical stress to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
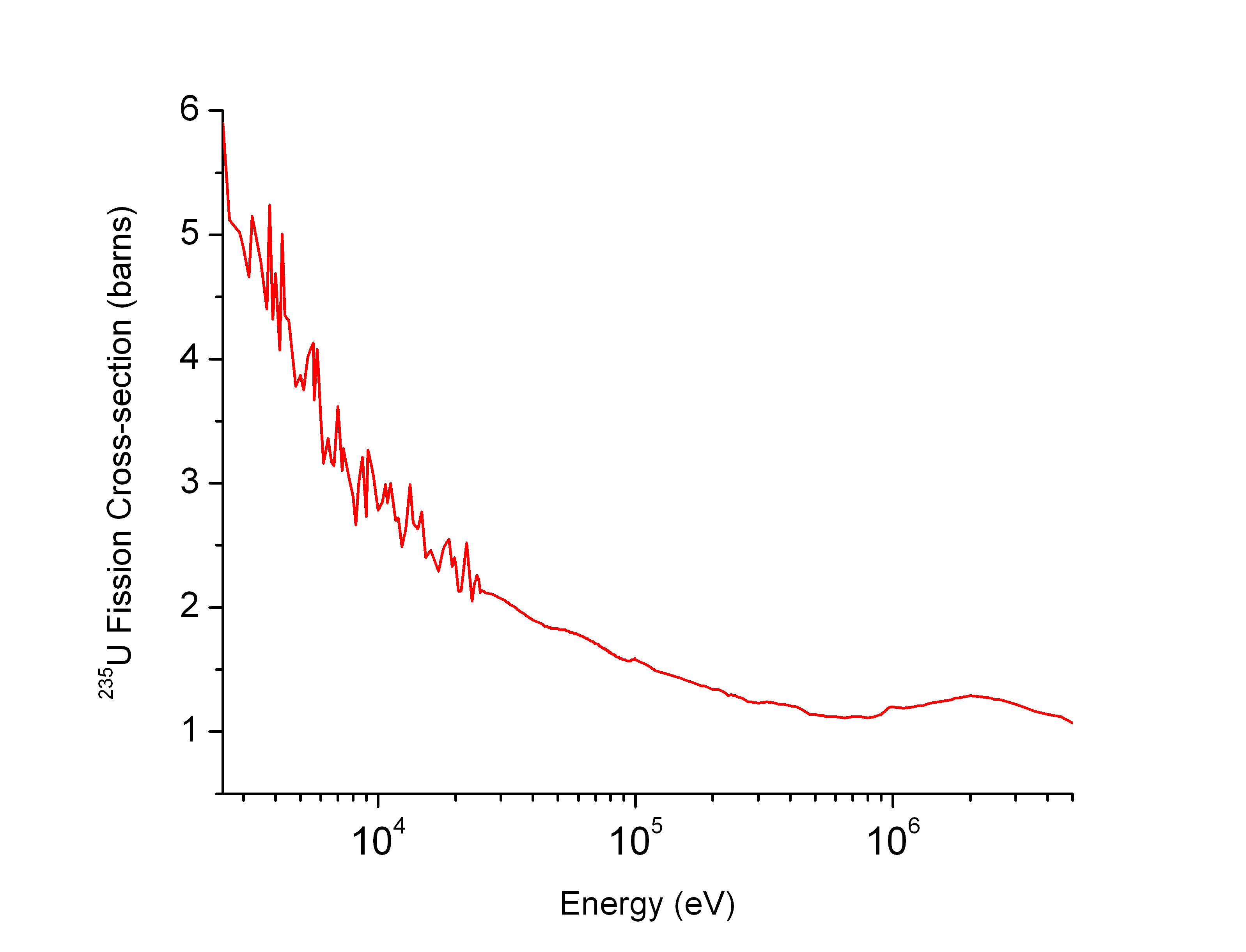
Neutron Cross Section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barn (unit)
A barn (symbol: b) is a metric unit of area equal to (100 fm2). This is equivalent to a square that is (10 fm) each side, or a circle of diameter approximately (11.28 fm). Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is also used in all fields of high-energy physics to express the cross sections of any scattering process, and is best understood as a measure of the probability of interaction between small particles. A barn is approximately the cross-sectional area of a uranium nucleus. The barn is also the unit of area used in nuclear quadrupole resonance and nuclear magnetic resonance to quantify the interaction of a nucleus with an electric field gradient. While the barn never was an SI unit, the SI standards body acknowledged it in the 8th SI Brochure (superseded in 2019) due to its use in particle physics. Etymology During Manhattan Project research on the atomic bomb dur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |