|
Xenon-135
Xenon-135 (135Xe) is an Isotope#Radioactive, primordial, and stable isotopes, unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison (2 million Barn (unit), barns; up to 3 million barn (unit), barns under reactor conditions), with a significant effect on nuclear reactor operation. The ultimate yield of xenon-135 from fission is 6.3%, though most of this is from fission-produced tellurium-135 and iodine-135. 135Xe effects on reactor restart In a typical nuclear reactor fueled with uranium-235, the presence of 135Xe as a fission product presents designers and operators with problems due to its large neutron cross section for absorption. Because absorbing neutrons can impair a nuclear reactor's ability to increase power, reactors are designed to mitigate this effect and operators are trained to anticipate and react to these transients. This practice dates to the Chicago ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant. The capture of neutrons by short half-life fission products is known as reactor poisoning; neutron capture by long-lived or stable fission products is called reactor slagging. Transient fission product poisons Some of the fission products generated during nuclear reactions have a high neutron absorption capacity, such as xenon-135 (microscopic cross-section σ = 2,000,000 barns (b); up to 3 million barns in reacto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons production and Research reactor, research. Fissile material, Fissile nuclei (primarily uranium-235 or plutonium-239) absorb single neutron, neutrons and split, releasing energy and multiple neutrons, which can induce further fission. Reactors stabilize this, regulating Neutron absorber, neutron absorbers and neutron moderator, moderators in the core. Fuel efficiency is exceptionally high; Enriched uranium#Low-enriched uranium (LEU), low-enriched uranium is 120,000 times more energy dense than coal. Heat from nuclear fission is passed to a working fluid Nuclear reactor#By coolant, coolant. In commercial reactors, this drives Turbine, turbines and electrical generator shafts. Some reactors are used for district heating, and isotopes, isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
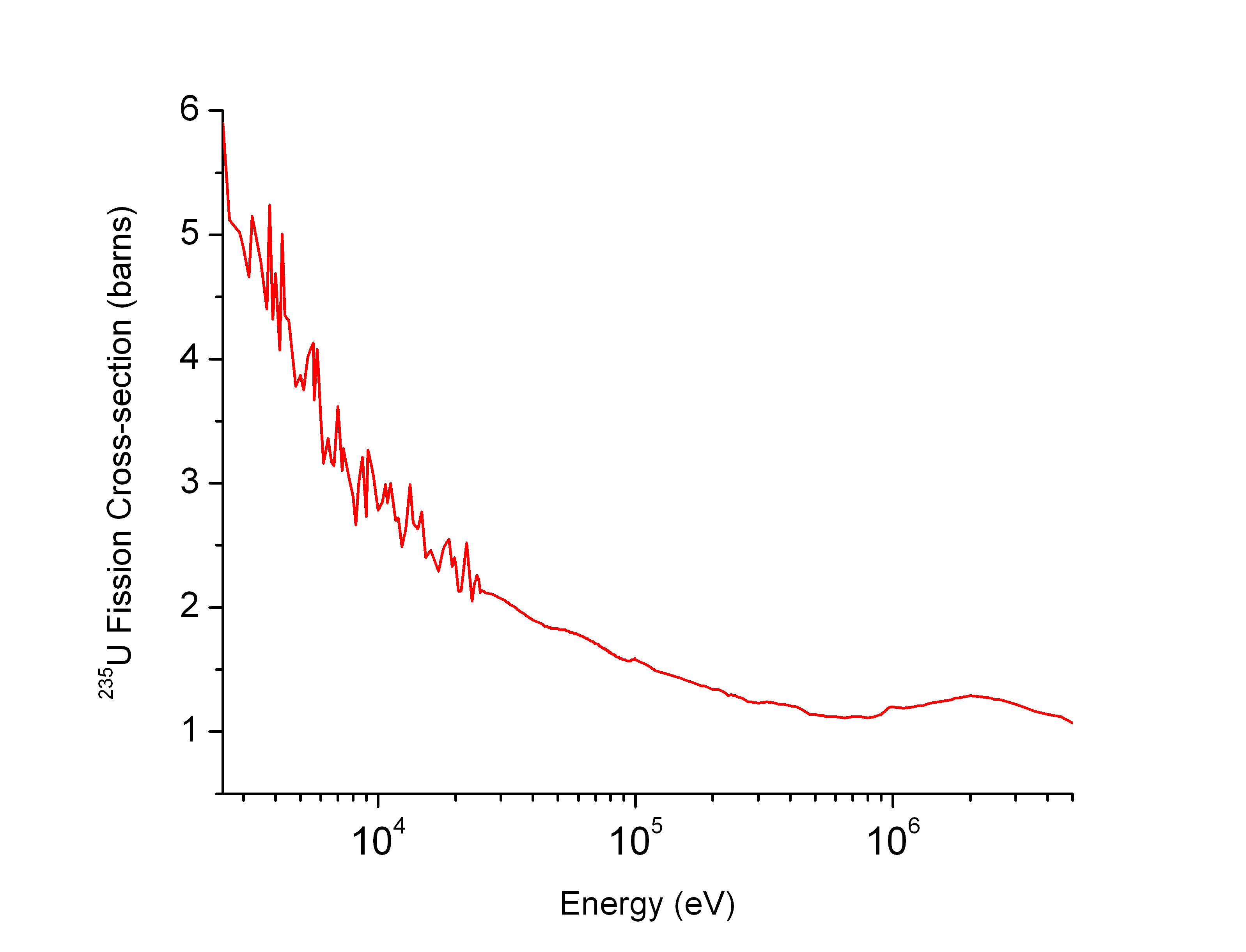
Xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-135
There are 40 known isotopes of iodine (53I) from 108I to 147I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element. Its longest-lived radioactive isotope, 129I, has a half-life of 16.14 million years, which is too short for it to exist as a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents. All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine - 123I, 124I, 125I, and 131I. All industrial use of radioactive iodine isotopes involves these four. The isotope 135I has a half-life less than seven hours, which is inconveniently shor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and additional gamma radiation that begins immediately after, even though this radiation is not produced directly by the fission even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manhattan Project
The Manhattan Project was a research and development program undertaken during World War II to produce the first nuclear weapons. It was led by the United States in collaboration with the United Kingdom and Canada. From 1942 to 1946, the project was directed by Major General Leslie Groves of the United States Army Corps of Engineers, U.S. Army Corps of Engineers. Nuclear physicist J. Robert Oppenheimer was the director of the Los Alamos Laboratory that designed the bombs. The Army program was designated the Manhattan District, as its first headquarters were in Manhattan; the name gradually superseded the official codename, Development of Substitute Materials, for the entire project. The project absorbed its earlier British counterpart, Tube Alloys, and subsumed the program from the American civilian Office of Scientific Research and Development. The Manhattan Project employed nearly 130,000 people at its peak and cost nearly US$2 billion (equivalent to about $ b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Cross Section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian and naturalized American physicist, renowned for being the creator of the world's first artificial nuclear reactor, the Chicago Pile-1, and a member of the Manhattan Project. He has been called the "architect of the nuclear age" and the "architect of the atomic bomb". He was one of very few physicists to excel in both theoretical physics, theoretical and experimental physics. Fermi was awarded the 1938 Nobel Prize in Physics for his work on induced radioactivity by neutron bombardment and for the discovery of transuranium elements. With his colleagues, Fermi filed several patents related to the use of nuclear power, all of which were taken over by the US government. He made significant contributions to the development of statistical mechanics, Quantum mechanics, quantum theory, and nuclear physics, nuclear and particle physics. Fermi's first major contribution involved the field of statistical mechanics. Afte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emilio Segrè
Emilio Gino Segrè ( ; ; 1 February 1905 – 22 April 1989) was an Italian-American nuclear physicist and radiochemist who discovered the elements technetium and astatine, and the antiproton, a subatomic antiparticle, for which he was awarded the Nobel Prize in Physics in 1959, along with Owen Chamberlain. Born in Tivoli, near Rome, Segrè studied engineering at the University of Rome La Sapienza before taking up physics in 1927. Segrè was appointed assistant professor of physics at the University of Rome in 1932 and worked there until 1936, becoming one of the Via Panisperna boys. From 1936 to 1938 he was director of the Physics Laboratory at the University of Palermo. After a visit to Ernest O. Lawrence's Berkeley Radiation Laboratory, he was sent a molybdenum strip from the laboratory's cyclotron accelerator in 1937, which was emitting anomalous forms of radioactivity. Using careful chemical and theoretical analysis, Segrè was able to prove that some of the radiat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chien-Shiung Wu
Chien-Shiung Wu ( zh, t=吳健雄, p=Wú Jiànxióng, w=Wu2 Chien4-Hsiung2; May 31, 1912 – February 16, 1997) was a Chinese-American particle physics, particle and experimental physicist who made significant contributions in the fields of nuclear physics, nuclear and particle physics. Wu worked on the Manhattan Project, where she helped develop the process for separating uranium into uranium-235 and uranium-238 isotopes by gaseous diffusion. She is best known for conducting the Wu experiment, which proved that Parity (physics), parity is not conservation law, conserved. This discovery resulted in her colleagues Tsung-Dao Lee and Chen-Ning Yang winning the 1957 Nobel Prize in Physics, while Wu herself was awarded the inaugural Wolf Prize in Physics in 1978. Her expertise in experimental physics evoked comparisons to Marie Curie. Her nicknames include the "First Lady of Physics", the "Chinese Marie Curie" and the "Queen of Nuclear Research". Early life Chien-Shiung Wu was born i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Radioisotope
A synthetic radioisotope is a radionuclide that is not found in nature: no natural process or mechanism exists which produces it, or it is so unstable that it decays away in a very short period of time. Frédéric Joliot-Curie and Irène Joliot-Curie were the first to produce a synthetic radioisotope in the 20th century. Examples include technetium-98 and promethium-146. Many of these are found in, and harvested from, spent nuclear fuel assemblies. Some must be manufactured in particle accelerators. Production Some synthetic radioisotopes are extracted from spent nuclear reactor fuel rods, which contain various fission products. For example, it is estimated that up to 1994, about 49,000 terabecquerels (78 metric tons) of technetium were produced in nuclear reactors; as such, anthropogenic technetium is far more abundant than technetium from natural radioactivity. Some synthetic isotopes are produced in significant quantities by fission but are not yet being reclaimed. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
B Reactor
The B Reactor at the Hanford Site, near Richland, Washington, was the first large-scale nuclear reactor ever built, at 250 MW. It achieved criticality on September 26, 1944. The project was a key part of the Manhattan Project, the United States nuclear weapons development program during World War II. Its purpose was to convert part of its natural uranium fuel into plutonium-239 by neutron activation, for use in nuclear weapons. Pure plutonium was then chemically separated in the site's T Plant, as an alternative to the Project's uranium enrichment plants. The B reactor was graphite moderated and water-cooled, via a contaminating open cycle with the Columbia River. It was preceded by Clinton Laboratory's X-10 Graphite Reactor, a pilot plant for reactor production and chemical separation of plutonium, which by mid-1944 had reached a capacity of 4 MW. The B reactor thus represented a massive leap of two orders of magnitude in reactor design. Primarily constructed by DuPont, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |