|
Uncoupler
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Because the intracellular supply of protons is replenished, uncouplers actually stimulate cellular metabolism and oxygen consumption (despite their inhibitory effects on oxidative phosphorylation) and increase the energy cost of generating ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes. Description Classical uncouplers have five properties: # the complete release of respiratory control # the substitution of all coupled processes ( ATP synthesis, transhydrogenation, reve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide Resistance Action Committee
The Insecticide Resistance Action Committee (IRAC) was formed in 1984 and works as a specialist technical group of the industry association CropLife to be able to provide a coordinated industry response to prevent or delay the development of insecticide resistance in insect, mite and nematode pests. IRAC strives to facilitate communication and education on insecticide and traits resistance as well as to promote the development and facilitate the implementation of insecticide resistance management strategies. IRAC is recognised by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) of the United Nations as an advisory body on matters pertaining to insecticide resistance. Pesticideresistance.org is a database financed by IRAC, US Department of Agriculture, and others. Sponsors IRAC's sponsors are: ADAMA, BASF, Bayer CropScience, Corteva, FMC, Mitsui Chemicals, Nihon Nohyaku, Sumitomo Chemical, Syngenta and UPL. Mode of action classification ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Cyanide-p-trifluoromethoxyphenylhydrazone
Carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (FCCP) is an ionophore that is a mobile ion carrier. It is referred to as an Uncoupler, uncoupling agent because it disrupts Adenosine triphosphate, ATP ATP synthase, synthesis by transporting hydrogen ions through the mitochondrial membrane before they can be used to provide the energy for oxidative phosphorylation. It is a nitrile and hydrazone. FCCP was first described in 1962 by Heytler. See also * Carbonyl cyanide m-chlorophenyl hydrazone, Carbonyl cyanide ''m''-chlorophenyl hydrazone (CCCP) References {{reflist Ionophores Nitriles Trifluoromethyl ethers Uncouplers Hydrazones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Cyanide M-chlorophenyl Hydrazone
Carbonyl cyanide ''m''-chlorophenyl hydrazone (CCCP; also known as ) is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and protonophore. In general, CCCP causes the gradual destruction of living cells and death of the organism, although mild doses inducing partial decoupling have been shown to increase median and maximum lifespan in '' C. elegans'' models, suggesting a degree of hormesis. CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. The chemical acts essentially as an ionophore and reduces the ability of ATP synthase to function optimally. It is routinely used as an experimental uncoupling agent in cell and molecular biology, particularly in the study of mitophagy, where it was integral in discovering the role of the Parkinson's disease-associated ubiquitin ligase Parkin. Outside of its effects on mitochondria, CCCP may also disrupt lysosomal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation (biochemistry), fermentation processes such as anaerobic glycolysis. The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle, producing carbon dioxide and the energetic reducing agent, electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to ATP synthase, produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a ser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BAM15
BAM15 is a novel mitochondrial protonophore uncoupler capable of protecting mammals from acute renal ischemic-reperfusion injury and cold-induced kidney tubule damage. It is being studied for the treatment of obesity sepsis Sepsis is a potentially life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs. This initial stage of sepsis is followed by suppression of the immune system. Common signs and s ..., and cancer. References {{reflist External links Fat-fighting molecule sees the body burn more fuel Oxadiazoles Pyrazines Bicyclic compounds 2-Fluorophenyl compounds Anilines Secondary amines Uncouplers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
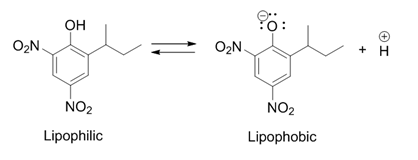
Dinitro-ortho-cresol
Dinitro-''ortho''-cresol (DNOC) is an organic compound with the structural formula CH3C6H2(NO2)2OH. It is a yellow solid that is only slightly soluble in water. It is extremely toxic to humans and was previously used as a herbicide and insecticide. Preparation This compound is prepared by disulfonation of ''o''-cresol. The resulting disulfonate is then treated with nitric acid to give DNOC. A variety of related derivatives are known including those where the methyl group is replaced by ''sec''-butyl (dinoseb), ''tert''-butyl ( dinoterb), and 1-methylheptyl ( dinocap). These are prepared by the direct nitration of the alkyphenols. Applications and safety DNOC is an uncoupler, which means that it interferes with adenosine triphosphate (ATP) production, making it extremely toxic to humans. DNOC was one of the earliest pesticides developed, being used as an insecticide since the 1890s and a herbicide since the 1930s. It was banned for use as a pesticide in the United States in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorfenapyr
Chlorfenapyr is an insecticide, and specifically a pro-insecticide (meaning it is metabolized into an active insecticide after entering the host). It is derived from a class of microbially produced compounds known as halogenated pyrroles. History and Applications Chlorfenapyr was developed by American Cyanamid from the natural product dioxapyrrolomycin, which was isolated from ''Streptomyces fumanus''. The United States Environmental Protection Agency initially denied registration in 2000 for use on cotton primarily because of concerns that the insecticide was toxic to birds and because effective alternatives were available. However, it was registered by the EPA in January, 2001 for use on non-food crops in greenhouses. In 2005, the EPA established a tolerance for residues of chlorfenapyr in or on all food commodities. Chlorfenapyr is also used as a wool insect-proofing agent, and was introduced as an alternative to synthetic pyrethroids due to a lower toxicity to mammalian a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinoseb
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity. It also finds use as a polymerisation inhibitor, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene and other unsaturated monomers when they are being purified by distillation. History In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as an insecticide. It was later also used as an herbicide and also fungicide after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dinoseb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinoterb
Dinoterb is a chemical compound previously used as a contact herbicide. It is an uncoupler, affecting respiration in mitochondria and photosynthesis in chloroplasts A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur .... It is banned for use in the European Union and use was suspended in the United States in 1986. References Links * Herbicides Dinitrophenol derivatives Tert-butyl compounds Uncouplers Group 24 herbicides {{alkanederivative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ellipticine
Ellipticine is a tetracyclic alkaloid first extracted from the tree species ''Ochrosia elliptica'' and ''Rauvolfia sandwicensis'' which inhibits the enzyme topoisomerase II via intercalation (biochemistry), intercalative binding to DNA. Natural occurrence and synthesis Ellipticine is an organic compound present in several trees within the genera ''Ochrosia'', ''Rauvolfia'', and ''Aspidosperma''. It was first isolated from ''Ochrosia elliptica'' Labill., a flowering tree native to Australia and New Caledonia which gives the alkaloid its name, in 1959, and synthesised by Robert Burns Woodward later the same year. Biological activity Ellipticine is a known intercalation (biochemistry), intercalator, capable of entering a DNA strand between base pairs. In its intercalated state, ellipticine binds strongly and lies parallel to the base pairs, increasing the DNA supercoil, superhelical density of the DNA. Intercalated ellipticine binds directly to topoiso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicoumarol
Dicoumarol ( INN) or dicumarol ( USAN) is a naturally occurring anticoagulant drug that depletes stores of vitamin K (similar to warfarin, a drug that dicoumarol inspired). It is also used in biochemical experiments as an inhibitor of reductases. Dicoumarol is a natural chemical substance of combined plant and fungal origin. It is a derivative of coumarin, a bitter-tasting but sweet-smelling substance made by plants that does not itself affect coagulation, but which is (classically) transformed in mouldy feeds or silages by a number of species of fungi, into active dicoumarol. Dicoumarol does affect coagulation, and was discovered in mouldy wet sweet-clover hay, as the cause of a naturally occurring bleeding disease in cattle. See warfarin for a more detailed discovery history. Identified in 1940, dicoumarol became the prototype of the 4-hydroxycoumarin anticoagulant drug class. Dicoumarol itself, for a short time, was employed as a medicinal anticoagulant drug, but since the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |