|
Troc
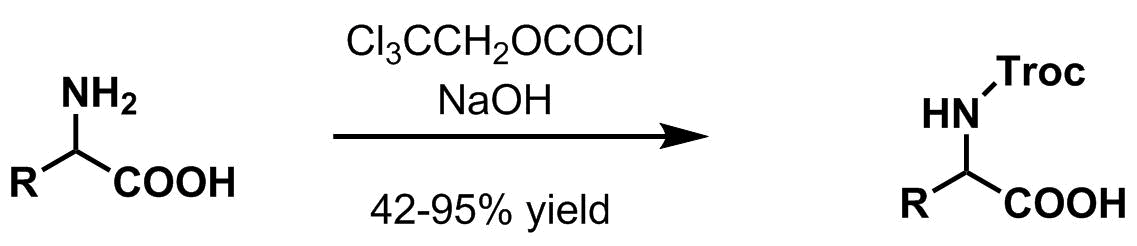
Trichloroethyl chloroformate is used in organic synthesis for the introduction of the trichloroethyl chloroformate (Troc) protecting group for amines, thiols and alcohols. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy. The troc group is traditionally removed ''via'' Zn insertion in the presence of acetic acid, resulting in elimination and decarboxylation. Amine protection – 2,2,2-Trichloroethoxycarbonyl (Troc) : 2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a protecting group for amines in organic synthesis. Most common amine protection methods * 2,2,2-Trichloroethyl chloroformate, pyridine or aqueous sodium hydroxide at ambient temperature : * Electrolysis : * Deprotection using zinc metal A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity". Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, William Nicholson and Anthony Carlisle sought to further Volt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troc Dep Mod Copy Q
Trichloroethyl chloroformate is used in organic synthesis for the introduction of the trichloroethyl chloroformate (Troc) protecting group for amines, thiols and alcohols. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy. The troc group is traditionally removed ''via'' Zn insertion in the presence of acetic acid, resulting in elimination and decarboxylation. Amine protection – 2,2,2-Trichloroethoxycarbonyl (Troc) : 2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a protecting group for amines in organic synthesis. Most common amine protection methods * 2,2,2-Trichloroethyl chloroformate, pyridine or aqueous sodium hydroxide at ambient temperature : * Electrolysis : * Deprotection using zinc metal A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multiste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troc Protecting Group
Trichloroethyl chloroformate is used in organic synthesis for the introduction of the trichloroethyl chloroformate (Troc) protecting group for amines, thiols and alcohols. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy. The troc group is traditionally removed ''via'' Zn insertion in the presence of acetic acid, resulting in elimination and decarboxylation. Amine protection – 2,2,2-Trichloroethoxycarbonyl (Troc) : 2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a protecting group for amines in organic synthesis. Most common amine protection methods * 2,2,2-Trichloroethyl chloroformate, pyridine or aqueous sodium hydroxide at ambient temperature : * Electrolysis : * Deprotection using zinc metal A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multiste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troc Group Protection
Trichloroethyl chloroformate is used in organic synthesis for the introduction of the trichloroethyl chloroformate (Troc) protecting group for amines, thiols and alcohols. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy. The troc group is traditionally removed ''via'' Zn insertion in the presence of acetic acid, resulting in elimination and decarboxylation. Amine protection – 2,2,2-Trichloroethoxycarbonyl (Troc) : 2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a protecting group for amines in organic synthesis. Most common amine protection methods * 2,2,2-Trichloroethyl chloroformate, pyridine or aqueous sodium hydroxide at ambient temperature : * Electrolysis : * Deprotection using zinc metal A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multiste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroformates
Chloroformates are a class of organic compounds with the formula ROC(O)Cl. They are formally esters of chloroformic acid. Most are colorless, volatile liquids that degrade in moist air. A simple example is methyl chloroformate, which is commercially available. Chloroformates are used as reagents in organic chemistry. For example, benzyl chloroformate is used to introduce the Cbz (carboxybenzyl) protecting group and fluorenylmethyloxycarbonyl chloride is used to introduce the FMOC protecting group. Chloroformates are popular in the field of chromatography as derivatization agents. They convert polar compounds into less polar more volatile derivatives. In this way, chloroformates enable relatively simple transformation of large array of metabolites (aminoacids, amines, carboxylic acids, phenols) for analysis by gas chromatography / mass spectrometry. Reactions The reactivity of chloroformates and acyl chlorides are similar. Representative reactions are: * Reaction with amines to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotection Using Zinc Metal
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |