|
Tetraarsenic Tetrasulfide
Realgar ( ), also known as "ruby sulphur" or "ruby of arsenic", is an arsenic sulfide mineral with the chemical formula α-. It is a soft, sectile mineral occurring in monoclinic crystals, or in granular, compact, or powdery form, often in association with the related mineral, orpiment (). It is orange-red in color, melts at 320 °C, and burns with a bluish flame releasing fumes of arsenic and sulfur. Realgar is soft with a Mohs hardness of 1.5 to 2 and has a specific gravity of 3.5. Its streak is orange colored. It is trimorphous with pararealgar and bonazziite. Its name comes from the Arabic ''rahj al-ġār'' (, "powder of the mine"), via Medieval Latin, and its earliest record in English is in the 1390s. Uses Realgar is a minor ore of arsenic extracted in China, Peru, and the Philippines. Historical uses Realgar was used by firework manufacturers to create the color white in fireworks prior to the availability of powdered metals such as aluminium, magnesium and tita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide Mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite *Chalcocite *Bornite *Galena *Sphalerite * Chalcopyrite * Pyrrhotite * Millerite * Pentlandite * Covellite * Cinnabar * Realgar *Orpiment * Stibnite *Pyrite * Marcasite *Molybdenite Sulfarsenides: *Cobaltite *Arsenopyrite * Gersdorffite Sulfosalts: * Pyrargyrite * Proustite *Tetrahedrite * Tennantite * Enargite * B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance with a relative density greater ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoarsenic Chemistry
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known. History 140px, Cacodyl (tetramethyldiarsine) was one of the first organoarsenic compounds. Surprising for an area now considered of minor importance, organoarsenic chemistry played a prominent role in the history of the field of chemistry. The oldest known organoarsenic compound, the foul smelling cacodyl was reported in "cacodyl" (1760) and is sometimes classified as the first synthetic organometallic compound. The compound Salvarsan was one of the first pharmaceuticals, earning a Nobel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cacodylic Acid
Cacodylic acid is an organoarsenic compound with the formula (CH3)2 AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water. Neutralization of cacodylic acid with base gives cacodylate salts, e.g. sodium cacodylate. They are potent herbicides. Cacodylic acid/sodium cacodylate is a buffering agent in the preparation and fixation of biological samples for electron microscopy. History In the 18th century it was found that combining and four equivalents of potassium acetate () gives a product called " Cadet's fuming liquid" which contains cacodyl oxide, and cacodyl, . Early research into "cacodyls" was reported by Robert Bunsen at the University of Marburg. Bunsen said of the compounds, "The smell of this body produces instantaneous tingling of the hands and feet, and even giddiness and insensibility... It is remarkable that when one is exposed to the smell of these compounds the tongue becomes covered with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rodent
Rodents (from Latin , 'to gnaw') are mammals of the order Rodentia (), which are characterized by a single pair of continuously growing incisors in each of the upper and lower jaws. About 40% of all mammal species are rodents. They are native to all major land masses except for New Zealand, Antarctica, and several oceanic islands, though they have subsequently been introduced to most of these land masses by human activity. Rodents are extremely diverse in their ecology and lifestyles and can be found in almost every terrestrial habitat, including human-made environments. Species can be arboreal, fossorial (burrowing), saltatorial/richochetal (leaping on their hind legs), or semiaquatic. However, all rodents share several morphological features, including having only a single upper and lower pair of ever-growing incisors. Well-known rodents include mice, rats, squirrels, prairie dogs, porcupines, beavers, guinea pigs, and hamsters. Rabbits, hares, and pikas, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insect
Insects (from Latin ') are pancrustacean hexapod invertebrates of the class Insecta. They are the largest group within the arthropod phylum. Insects have a chitinous exoskeleton, a three-part body ( head, thorax and abdomen), three pairs of jointed legs, compound eyes and one pair of antennae. Their blood is not totally contained in vessels; some circulates in an open cavity known as the haemocoel. Insects are the most diverse group of animals; they include more than a million described species and represent more than half of all known living organisms. The total number of extant species is estimated at between six and ten million; In: potentially over 90% of the animal life forms on Earth are insects. Insects may be found in nearly all environments, although only a small number of species reside in the oceans, which are dominated by another arthropod group, crustaceans, which recent research has indicated insects are nested within. Nearly all insects hatch f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weed
A weed is a plant considered undesirable in a particular situation, "a plant in the wrong place", or a plant growing where it is not wanted.Harlan, J. R., & deWet, J. M. (1965). Some thoughts about weeds. ''Economic botany'', ''19''(1), 16-24. This introduces the concept of humans and their goals in a particular setting.Holzner, W., & Numata, M. (Eds.). (2013). ''Biology and ecology of weeds'' (Vol. 2). Springer Science & Business Media. The concept of weeds is particularly significant in agriculture, where the aim is growing crops or pastures of a single species, or a mixture of a few desired species. In such environments, other plant species are considered undesirable and therefore a weed. Besides, some weeds have undesirable characteristics making them a plant pest in most human settings.Harlan, J. R., & deWet, J. M. (1965). Some thoughts about weeds. ''Economic botany'', ''19''(1), 16-24.Holzner, W., & Numata, M. (Eds.). (2013). ''Biology and ecology of weeds'' (Vol. 2). Spri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chlorate
Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used * in fireworks, propellants and explosives, * to prepare oxygen, both in the lab and in chemical oxygen generators, * as a disinfectant, for example in medical mouthwashes, * in agriculture as an herbicide. Production On the industrial scale, potassium chlorate is produced by the salt metathesis reaction of sodium chlorate and potassium chloride: : NaClO3 + KCl → NaCl + KClO3 The reaction is driven by the low solubility of potassium chlorate in water. The equilibrium of the reaction is shifted to the right hand side by the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
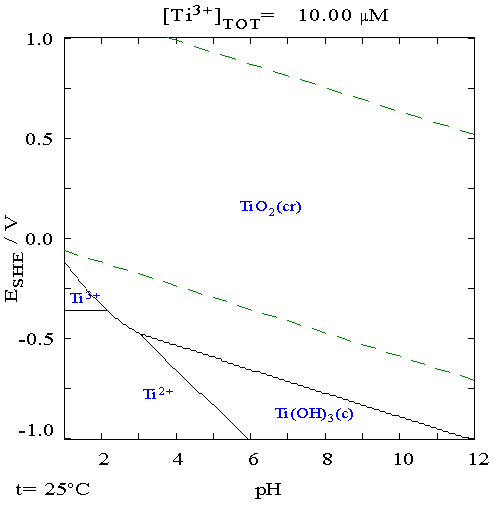
Titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Kingdom of Great Britain, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titan (mythology), Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll process, Kroll and Hunter process, Hunter processes. The most common compound, titanium dioxide, is a popular photocatalysis, photocatalyst and is used in the manufacture of white pigments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medieval Latin
Medieval Latin was the form of Literary Latin used in Roman Catholic Western Europe during the Middle Ages. In this region it served as the primary written language, though local languages were also written to varying degrees. Latin functioned as the main medium of scholarly exchange, as the liturgical language of the Church, and as the working language of science, literature, law, and administration. Medieval Latin represented a continuation of Classical Latin and Late Latin, with enhancements for new concepts as well as for the increasing integration of Christianity. Despite some meaningful differences from Classical Latin, Medieval writers did not regard it as a fundamentally different language. There is no real consensus on the exact boundary where Late Latin ends and Medieval Latin begins. Some scholarly surveys begin with the rise of early Ecclesiastical Latin in the middle of the 4th century, others around 500, and still others with the replacement of written Late La ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |