|
Trehalulose
Trehalulose is a disaccharide made up of a molecule of fructose bound to a molecule of glucose. Like isomaltulose, it is a structural isomer of sucrose that is present in small quantities in honey. It makes up 50% of sugars in the honeydew of silverleaf whiteflies and is synthesised from sucrose by some bacteria, such as ''Protaminombacter rubrum''. Because the anomeric carbon of the fructose moiety is not involved in the glycosidic bond, it is a reducing sugar. Physiology Because the fructose and glucose molecules are linked by a 1,1 glycosidic bond, which is more stable than the 1,2 glycosidic bond in sucrose, it is broken down more slowly than sucrose in the small intestine, giving it a lower glycemic index. This more stable bond also means that it cannot be utilised by ''Streptococcus mutans'', and it is therefore non-cariogenic. Properties Unlike isomaltulose, trehalulose strongly resists crystallisation, and forms an amorphous solid when dried. Its sweetness relati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stingless Bee
Stingless bees, sometimes called stingless honey bees or simply meliponines, are a large group of bees (about 550 described species), comprising the tribe Meliponini (or subtribe Meliponina according to other authors). They belong in the family Apidae, and are closely related to common honey bees, carpenter bees, orchid bees, and bumblebees. Meliponines have stingers, but they are highly reduced and cannot be used for defense, though these bees exhibit other defensive behaviors and mechanisms. Meliponines are not the only type of bee incapable of stinging: all male bees and many female bees of several other families, such as Andrenidae, also cannot sting. Some stingless bees have powerful mandibles and can inflict painful bites. Geographical distribution Stingless bees can be found in most tropical or subtropical regions of the world, such as Australia, Africa, Southeast Asia, and tropical America.Michener, C D. ''The bees of the World''. Johns Hopkins University Press, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose. Disaccharides are one of the four chemical groupings of carbohydrates ( monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule. The joining of monosaccharides into a double sugar happens by a condensation reaction, which involves the elimination of a water molecule from the functional groups only. Breaking apart a double sugar into its two monosaccharides is accomplished by hydrolysis with the help of a type of enzyme called a disaccharidase. As building the larger sugar ejects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidic Bond
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate. A glycosidic bond is formed between the hemiacetal or hemiketal group of a saccharide (or a molecule derived from a saccharide) and the hydroxyl group of some compound such as an alcohol. A substance containing a glycosidic bond is a glycoside. The term 'glycoside' is now extended to also cover compounds with bonds formed between hemiacetal (or hemiketal) groups of sugars and several chemical groups other than hydroxyls, such as -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Particularly in naturally occurring glycosides, the compound ROH from which the carbohydrate residue has been removed is often termed the aglycone, and the carbohydrate residue itself is sometimes referred to as the 'glycone'. S-, N-, C-, and O-glycosidic bonds Glyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Queensland
, mottoeng = By means of knowledge and hard work , established = , endowment = A$224.3 million , budget = A$2.1 billion , type = Public research university , chancellor = Peter Varghese , vice_chancellor = Deborah Terry , city = Brisbane, Queensland, Australia , students = 55,305 (2019) , undergrad = 35,051 (2019) , postgrad = 19,939 (2019) , faculty = 2,854 , campus = Multiple sites , colours = Purple , affiliations = Group of Eight Universitas 21 ASAIHL EdX , website = , logo = Logo of the University of Queensland.svg , coor = The University of Queensland (UQ, or Queensland University) is a public research university located primarily in Brisbane, the capital city of the Australian state of Queensland. Founded in 1909 by the Queensland parliament, UQ is one of the six sandstone universities, an informal designation of the oldest university in each state. As per 2023, The University of Queensland is ranked as 2nd in Australia and 42nd in the world ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Rotation
In chemistry, specific rotation ( �'') is a property of a chiral chemical compound. It is defined as the change in orientation of monochromatic plane-polarized light, per unit distance–concentration product, as the light passes through a sample of a compound in solution. Compounds which rotate the plane of polarization of a beam of plane polarized light clockwise are said to be dextrorotary, and correspond with positive specific rotation values, while compounds which rotate the plane of polarization of plane polarized light counterclockwise are said to be levorotary, and correspond with negative values. If a compound is able to rotate the plane of polarization of plane-polarized light, it is said to be “ optically active”. Specific rotation is an intensive property, distinguishing it from the more general phenomenon of optical rotation. As such, the ''observed'' rotation (α) of a sample of a compound can be used to quantify the enantiomeric excess of that compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal. Etymology The term comes from the Greek ''a'' ("without"), and ''morphé'' ("shape, form"). In some older articles and books, the term was used synonymously with glass. Today, "glassy solid" or "amorphous solid" is considered the overarching concept. Polymers are often amorphous. Structure Amorphous materials have an internal structure comprising interconnected structural blocks that can be similar to the basic structural units found in the corresponding crystalline phase of the same compound. Unlike crystalline materials, however, no long-range order exists. Localized order in amorphous materials can be categorized as short or medium range order. By convention, short range order extends only to the nearest neighbor shell, typically only 1-2 atomic spacings. Medium range order is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cariogenic
Tooth decay, also known as cavities or caries, is the breakdown of teeth due to acids produced by bacteria. The cavities may be a number of different colors from yellow to black. Symptoms may include pain and difficulty with eating. Complications may include inflammation of the tissue around the tooth, tooth loss and infection or abscess formation. The cause of cavities is acid from bacteria dissolving the hard tissues of the teeth ( enamel, dentin and cementum). The acid is produced by the bacteria when they break down food debris or sugar on the tooth surface. Simple sugars in food are these bacteria's primary energy source and thus a diet high in simple sugar is a risk factor. If mineral breakdown is greater than build up from sources such as saliva, caries results. Risk factors include conditions that result in less saliva such as: diabetes mellitus, Sjögren syndrome and some medications. Medications that decrease saliva production include antihistamines and antidepre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptococcus Mutans
''Streptococcus mutans'' is a facultatively anaerobic, gram-positive coccus (round bacterium) commonly found in the human oral cavity and is a significant contributor to tooth decay. It is part of the "streptococci" (plural, non-italic lowercase), an informal general name for all species in the genus ''Streptococcus''. The microbe was first described by James Kilian Clarke in 1924. This bacterium, along with the closely related species '' Streptococcus sobrinus'', can cohabit the mouth: Both contribute to oral disease, and the expense of differentiating them in laboratory testing is often not clinically necessary. Therefore, for clinical purposes they are often considered together as a group, called the mutans streptococci (plural, non-italic due to its being an informal group name). This grouping of similar bacteria with similar tropism can also be seen in the viridans streptococci, another group of ''Streptococcus'' species. Ecology ''S. mutans'' is naturally present in the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycemic Index
The glycemic (glycaemic) index (GI; ) is a number from 0 to 100 assigned to a food, with pure glucose arbitrarily given the value of 100, which represents the relative rise in the blood glucose level two hours after consuming that food. The GI of a specific food depends primarily on the quantity and type of carbohydrate it contains, but is also affected by the amount of entrapment of the carbohydrate molecules within the food, the fat and protein content of the food, the amount of organic acids (or their salts) in the food, and whether it is cooked and, if so, how it is cooked. GI tables, which list many types of foods and their GIs, are available. A food is considered to have a ''low GI'' if it is 55 or less; ''high GI'' if 70 or more; and ''mid-range GI'' if 56 to 69. The term was introduced in 1981 by David J. Jenkins and co-workers. It is useful for quantifying the relative rapidity with which the body breaks down carbohydrates. It takes into account only the available carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
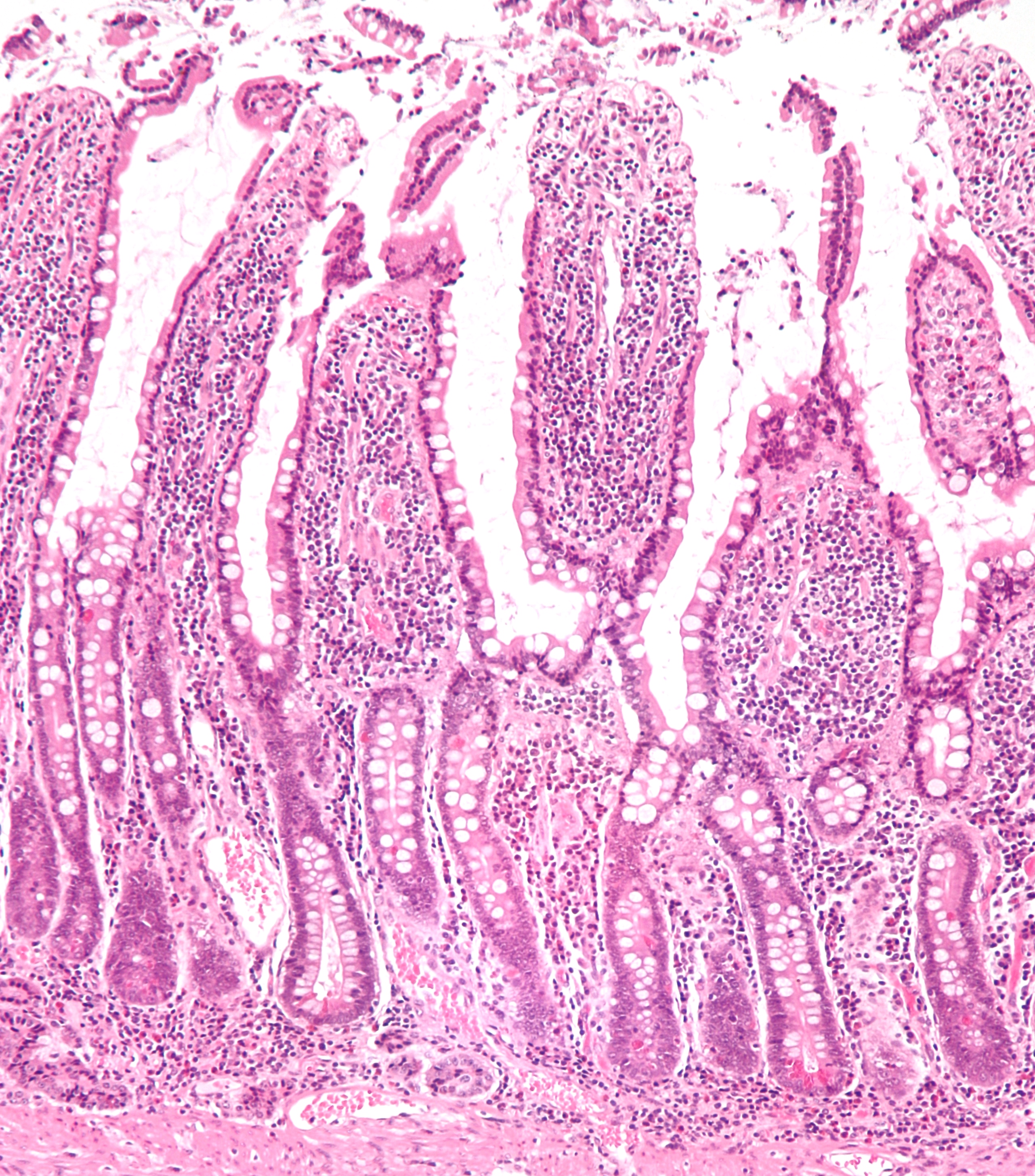
Small Intestine
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion. The small intestine is about long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is narrower in diameter. The small intestine has three distinct regions – the duodenum, jejunum, and ileum. The duodenum, the shortest, is where preparation for absorption through small finger-like protrusions called villi begins. The jejunum is specialized for the absorption through its lining by enterocytes: small nutrient particles which have been previously digested by enzymes in the duodenum. The main function of the ileum is to absorb vitamin B12, bile salts, and whatever products of digestion that were not absorbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. The monosaccharides can be divided into two groups: the aldoses, which have an aldehyde group, and the ketoses, which have a ketone group. Ketoses must first tautomerize to aldoses before they can act as reducing sugars. The common dietary monosaccharides galactose, glucose and fructose are all reducing sugars. Disaccharides are formed from two monosaccharides and can be classified as either reducing or nonreducing. Nonreducing disaccharides like sucrose and trehalose have glycosidic bonds between their anomeric carbons and thus cannot convert to an open-chain form with an al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |