|
Transferrin Receptor-2
Transferrin receptor 2 (TfR2) is a protein that in humans is encoded by the ''TFR2'' gene. This protein is involved in the uptake of transferrin-bound iron into cells by endocytosis, although its role is minor compared to transferrin receptor 1. Function This gene is a member of the transferrin receptor-like family and encodes a single-pass type II membrane protein with a protease associated (PA) domain, an M28 peptidase domain and a transferrin receptor-like dimerization domain. This protein mediates cellular uptake of transferrin-bound iron and mutations in this gene have been associated with hereditary hemochromatosis type III. Alternatively spliced variants which encode different protein isoforms have been described; however, not all variants have been fully characterized. See also * Transferrin receptor 1 * Transferrin Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and non-coding genes. During gene expression (the synthesis of Gene product, RNA or protein from a gene), DNA is first transcription (biology), copied into RNA. RNA can be non-coding RNA, directly functional or be the intermediate protein biosynthesis, template for the synthesis of a protein. The transmission of genes to an organism's offspring, is the basis of the inheritance of phenotypic traits from one generation to the next. These genes make up different DNA sequences, together called a genotype, that is specific to every given individual, within the gene pool of the population (biology), population of a given species. The genotype, along with environmental and developmental factors, ultimately determines the phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferrin
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Iron(III), Fe3+ ions. Human transferrin is encoded by the ''TF'' gene and produced as a 76 Dalton (unit), kDa glycoprotein. Transferrin glycoproteins bind iron tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of total body iron, it forms the most vital iron pool with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 atomic mass unit, kDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high (association constant is 1020 M−1 at pH 7.4) but decreases progressively with decreasing pH below neutrality. Transferrins are not limited to only binding to iron but also to different metal ions. These glycoproteins are located in various b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endocytosis
Endocytosis is a cellular process in which Chemical substance, substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a Vesicle (biology and chemistry), vesicle containing the ingested materials. Endocytosis includes pinocytosis (cell drinking) and phagocytosis (cell eating). It is a form of active transport. History The term was proposed by Christian de Duve, De Duve in 1963. Phagocytosis was discovered by Élie Metchnikoff in 1882. Pathways Endocytosis pathways can be subdivided into four categories: namely, receptor-mediated endocytosis (also known as clathrin-mediated endocytosis), caveolae, pinocytosis, and phagocytosis. * Clathrin-mediated endocytosis is mediated by the production of small (approx. 100 nm in diameter) vesicles that have a morphologically characteristic coat made up of the cytosolic protein clathrin. Clathrin-coated vesicles (CCVs) are found in vir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferrin Receptor 1
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the ''TF'' gene and produced as a 76 kDa glycoprotein. Transferrin glycoproteins bind iron tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of total body iron, it forms the most vital iron pool with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 kDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high ( association constant is 1020 M−1 at pH 7.4) but decreases progressively with decreasing pH below neutrality. Transferrins are not limited to only binding to iron but also to different metal ions. These glycoproteins are located in various bodily fluids of vertebrates. Some inve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferrin Receptor
Transferrin receptor (TfR) is a carrier protein for transferrin. It is needed for the import of iron into cells and is regulated in response to intracellular iron concentration. It imports iron by internalizing the transferrin-iron complex through receptor-mediated endocytosis.Figure 3: The cycle of transferrin and transferrin receptor 1-mediated cellular iron uptake./ref> The existence of a receptor for transferrin iron uptake has been recognized since the late 1950s. Earlier two transferrin receptors in humans, transferrin receptor 1 and transferrin receptor 2 had been characterized and until recently cellular iron uptake was believed to occur chiefly via these two well documented transferrin receptors. Both these receptors are transmembrane glycoproteins. TfR1 is a high affinity ubiquitously expressed receptor while expression of TfR2 is restricted to certain cell types and is unaffected by intracellular iron concentrations. TfR2 binds to transferrin with a 25-30 fold lower aff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemochromatosis
Iron overload is the abnormal and increased accumulation of total iron in the body, leading to organ damage. The primary mechanism of organ damage is oxidative stress, as elevated intracellular iron levels increase free radical formation via the Fenton reaction. Iron overload is often ''primary'' (i.e hereditary haemochromatosis, aceruloplasminemia) but may also be ''secondary'' to other causes (i.e. transfusional iron overload). Iron deposition most commonly occurs in the liver, pancreas, skin, heart, and joints. People with iron overload classically present with the triad of liver cirrhosis, secondary diabetes mellitus, and bronze skin. However, due to earlier detection nowadays, symptoms are often limited to general chronic malaise, arthralgia, and hepatomegaly. Signs and symptoms Organs most commonly affected by hemochromatosis include the liver, heart, and endocrine glands. Hemochromatosis may present with the following clinical syndromes: * liver: chronic liver disea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferrin Receptor 1
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the ''TF'' gene and produced as a 76 kDa glycoprotein. Transferrin glycoproteins bind iron tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of total body iron, it forms the most vital iron pool with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 kDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high ( association constant is 1020 M−1 at pH 7.4) but decreases progressively with decreasing pH below neutrality. Transferrins are not limited to only binding to iron but also to different metal ions. These glycoproteins are located in various bodily fluids of vertebrates. Some inve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
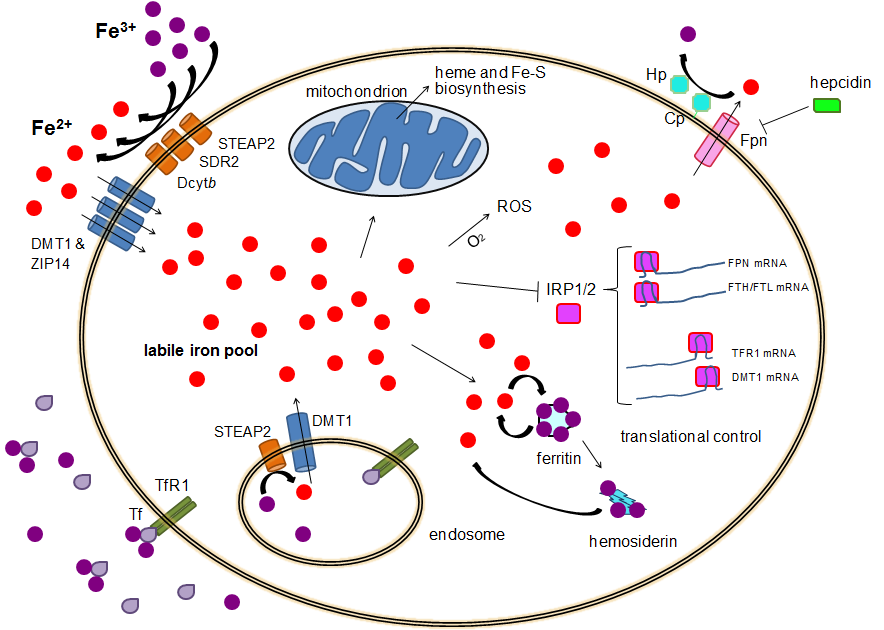
Iron Metabolism
Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia. Importance of iron regulation Iron is an essential bioelement for most forms of life, from bacteria to mammals. Its importance lies in its ability to mediate electron transfer. In the ferrous state (Fe2+), iron acts as an electron donor, while in the ferric state (Fe3+) it acts as an acceptor. Thus, iron plays a vital role in the cata ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |