|
Top-down Proteomics
Top-down proteomics is a method of protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... identification that either uses an ion trapping mass spectrometer to store an isolated protein ion for mass measurement and tandem mass spectrometry (MS/MS) analysis or other protein purification methods such as two-dimensional gel electrophoresis in conjunction with MS/MS. Top-down proteomics is capable of identifying and quantitating unique proteoforms through the analysis of intact proteins. The name is derived from the similar approach to DNA sequencing. During mass spectrometry intact proteins are typically ionized by electrospray ionization and trapped in a Fourier transform ion cyclotron resonance ( Penning trap), quadrupole ion trap (Paul trap) or Orbitrap mass spectrometer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Top-down Vs Bottom-up Proteomics Image
Top-down may refer to: Arts and entertainment * "Top Down", a 2007 song by Swizz Beatz * "Top Down", a song by Lil Yachty from ''Lil Boat 3'' * "Top Down", a song by Fifth Harmony from '' Reflection'' * "Topdown", a song by Channel Tres from the ''Channel Tres'' EP Science * Top-down reading, is a part of reading science that explains the reader's psycholinguistic strategies in using grammatical and lexical knowledge for comprehension rather than linearly decoding texts. * Top-down proteomics, a method for protein analysis * Top-down effects, effects of population density on a resource in a soil food web *Neural top–down control of physiology *Top-down processing, in Pattern recognition (psychology) Computing * Top-down and bottom-up design of information ordering * Top-down parsing, a parsing strategy beginning at the highest level of the parse tree **Top-down parsing language, an analytic formal grammar to study top-down parsers * Top-down perspective, a camera angle in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbitrap
In mass spectrometry, Orbitrap is an ion trap mass analyzer consisting of an outer barrel-like electrode and a coaxial inner spindle-like electrode that traps ions in an orbital motion around the spindle. The image current from the trapped ions is detected and converted to a mass spectrum by first using the Fourier transform of time domain of the harmonic to create a frequency signal which is converted to mass. History The concept of electrostatically trapping ions in an orbit around a central spindle was developed by Kenneth Hay Kingdon in the early 1920s. The Kingdon trap consists of a thin central wire and an outer cylindrical electrode. A static applied voltage results in a radial logarithmic potential between the electrodes. In 1981, Knight introduced a modified outer electrode that included an axial quadrupole term that confines the ions on the trap axis. Neither the Kingdon nor the Knight configurations were reported to produce mass spectra. In 1986, Professor Yuri Konst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tandem Mass Spectrometry
Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more stages of analysis using one or more mass analyzer are performed with an additional reaction step in between these analyses to increase their abilities to analyse chemical samples. A common use of tandem MS is the analysis of biomolecules, such as proteins and peptides. The molecules of a given sample are ionized and the first spectrometer (designated MS1) separates these ions by their mass-to-charge ratio (often given as m/z or m/Q). Ions of a particular m/z-ratio coming from MS1 are selected and then made to split into smaller fragment ions, e.g. by collision-induced dissociation, ion-molecule reaction, or photodissociation. These fragments are then introduced into the second mass spectrometer (MS2), which in turn separates the fragments by their m/z-ratio and detects them. The fragmentation step makes it possible to identify and separate ions that have very simil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shotgun Proteomics
Shotgun proteomics refers to the use of bottom-up proteomics techniques in identifying proteins in complex mixtures using a combination of high performance liquid chromatography combined with mass spectrometry. The name is derived from shotgun sequencing of DNA which is itself named after the rapidly expanding, quasi-random firing pattern of a shotgun. The most common method of shotgun proteomics starts with the proteins in the mixture being digested and the resulting peptides are separated by liquid chromatography. Tandem mass spectrometry is then used to identify the peptides. Targeted proteomics using SRM and data-independent acquisition methods are often considered alternatives to shotgun proteomics in the field of bottom-up proteomics. While shotgun proteomics uses data-dependent selection of precursor ions to generate fragment ion scans, the aforementioned methods use a deterministic method for acquisition of fragment ion scans. History Shotgun proteomics arose from the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Mass Spectrometry
Protein mass spectrometry refers to the application of mass spectrometry to the study of proteins. Mass spectrometry is an important method for the accurate mass determination and characterization of proteins, and a variety of methods and instrumentations have been developed for its many uses. Its applications include the identification of proteins and their post-translational modifications, the elucidation of protein complexes, their subunits and functional interactions, as well as the global measurement of proteins in proteomics. It can also be used to localize proteins to the various organelles, and determine the interactions between different proteins as well as with membrane lipids. The two primary methods used for the ionization of protein in mass spectrometry are electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI). These ionization techniques are used in conjunction with mass analyzers such as tandem mass spectrometry. In general, the prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bottom-up Proteomics
Bottom-up proteomics is a common method to identify proteins and characterize their amino acid sequences and post-translational modifications by proteolytic digestion of proteins prior to analysis by mass spectrometry. BUP techniques can be an alternative to Maldi-Tof MS approaches, as they allow the identification of bacterial strains and the characterization of potential resistance and virulence factors in a single run. The major alternative workflow used in proteomics is called top-down proteomics where intact proteins are purified prior to digestion and/or fragmentation either within the mass spectrometer or by 2D electrophoresis. Essentially, bottom-up proteomics is a relatively simple and reliable means of determining the protein make-up of a given sample of cells, tissues, etc. In bottom-up proteomics, the crude protein extract is enzymatically digested, followed by one or more dimensions of separation of the peptides by liquid chromatography coupled to mass spectrometry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Data Normalization
In mathematics and computer science, a canonical, normal, or standard form of a mathematical object is a standard way of presenting that object as a mathematical expression. Often, it is one which provides the simplest representation of an object and allows it to be identified in a unique way. The distinction between "canonical" and "normal" forms varies from subfield to subfield. In most fields, a canonical form specifies a ''unique'' representation for every object, while a normal form simply specifies its form, without the requirement of uniqueness. The canonical form of a positive integer in decimal representation is a finite sequence of digits that does not begin with zero. More generally, for a class of objects on which an equivalence relation is defined, a canonical form consists in the choice of a specific object in each class. For example: *Jordan normal form is a canonical form for matrix similarity. *The row echelon form is a canonical form, when one considers as equ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
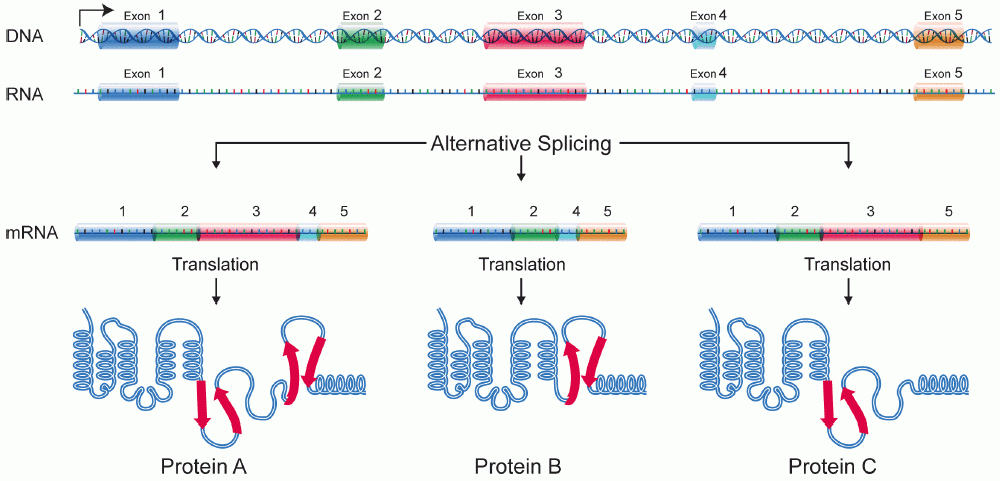
Protein Isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from Alternative splicing, alternative splicings, variable Promoter (genetics), promoter usage, or other Post-transcriptional modification, post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments (Exon, exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions of genes revealed by the human genome project ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionization, ionized, for example by bombarding it with a Electron ionization, beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron-transfer Dissociation
Electron-transfer dissociation (ETD) is a method of Fragmentation (mass spectrometry), fragmenting multiply-charged gaseous macromolecules in a mass spectrometer between the stages of tandem mass spectrometry (MS/MS). Similar to electron-capture dissociation, ETD induces fragmentation of large, multiply-charged cations by transferring electrons to them. ETD is used extensively with polymers and biological molecules such as proteins and peptides for sequence analysis. Transferring an electron causes Structure#Biological, peptide backbone cleavage into Peptide sequence tag, c- and z-ions while leaving Lability, labile Post-translational modification, post translational modifications (PTM) intact. The technique only works well for higher charge state peptide or polymer ions (z>2). However, relative to collision-induced dissociation (CID), ETD is advantageous for the fragmentation of longer peptides or even entire proteins. This makes the technique important for top-down proteomics. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron-capture Dissociation
Electron-capture dissociation (ECD) is a method of fragmenting gas-phase ions for structure elucidation of peptides and proteins in tandem mass spectrometry. It is one of the most widely used techniques for activation and dissociation of mass selected precursor ion in MS/MS. It involves the direct introduction of low-energy electrons to trapped gas-phase ions. History Electron-capture dissociation was developed by Roman Zubarev and Neil Kelleher while in Fred McLafferty's lab at Cornell University. Irradiation of melittin 4+ ions and ubiquitin 10+ ions (trapped in FT-MS cell) by laser pulses not only resulted in peculiar c', z fragmentation but also charge reduction. It was suggested that if FT cell is modified to trap cations and electrons simultaneously, secondary electrons emitted by UV photons increases the charge reduction effect and c′, z• fragmentation. Replacing UV laser with EI source led to the development of this new technique. Principles Electron-capture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragmentation (mass Spectrometry)
In mass spectrometry, fragmentation is the dissociation of energetically unstable molecular ions formed from passing the molecules mass spectrum. These reactions are well documented over the decades and fragmentation patterns are useful to determine the molar weight and structural information of unknown molecules. Fragmentation that occurs in tandem mass spectrometry experiments has been a recent focus of research, because this data helps facilitate the identification of molecules. Mass spectrometry techniques Fragmentation can occur in the ion source (in-source fragmentation) where it has been used with electron ionization to help identify molecules and, recently (2020), with electrospray ionization it has been shown to provide the same benefit in facilitating molecular identification. Prior to these experiments, electrospray ionization in-source fragmentation was generally considered an undesired effect however, electrospray ionization using Enhanced In-Source Fragmentation/Ann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |