|
Titanium Disulfide
Titanium disulfide is an inorganic compound with the formula Ti S2. A golden yellow solid with high electrical conductivity, it belongs to a group of compounds called transition metal di chalcogenides, which consist of the stoichiometry M E2. TiS2 has been employed as a cathode material in rechargeable batteries. Structure With a layered structure, TiS2 adopts a hexagonal close packed (hcp) structure, analogous to cadmium iodide (CdI2). In this motif, half of the octahedral holes are filled with a "cation", in this case Ti4+. Each Ti centre is surrounded by six sulfide ligands in an octahedral structure. Each sulfide is connected to three Ti centres, the geometry at S being pyramidal. Several metal di chalcogenides adopt similar structures, but some, notably MoS2, do not. The layers of TiS2 consist of covalent Ti-S bonds. The individual layers of TiS2 are bound together by van der Waals forces, which are relatively weak intermolecular forces. It crystallises in the space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°. Regular hexagon A regular hexagon is defined as a hexagon that is both equilateral and equiangular. In other words, a hexagon is said to be regular if the edges are all equal in length, and each of its internal angle is equal to 120°. The Schläfli symbol denotes this polygon as \ . However, the regular hexagon can also be considered as the cutting off the vertices of an equilateral triangle, which can also be denoted as \mathrm\ . A regular hexagon is bicentric, meaning that it is both cyclic (has a circumscribed circle) and tangential (has an inscribed circle). The common length of the sides equals the radius of the circumscribed circle or circumcircle, which equals \tfrac times the apothem (radius of the inscribed circle). Measurement The longest diagonal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit very different physical property, physical or list of materials properties#Mechanical properties, mechanical properties when measured along different axes, e.g. absorbance, refractive index, electrical resistivity and conductivity, conductivity, and tensile strength. An example of anisotropy is light coming through a polarizer. Another is wood, which is easier to split along its wood grain, grain than across it because of the directional non-uniformity of the grain (the grain is the same in one direction, not all directions). Fields of interest Computer graphics In the field of computer graphics, an anisotropic surface changes in appearance as it rotates about its normal (geometry), geometric normal, as is the case with velvet. Anisotropic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angstrom
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). It was originally spelled with Swedish letters, as Ångström and later as ångström (). The latter spelling is still listed in some dictionaries, but is now rare in English texts. Some popular US dictionaries list only the spelling ''angstrom''. The unit's symbol is Å, which is a letter of the Swedish alphabet, regardless of how the unit is spelled. However, "A" or "A.U." may be used in less formal contexts or typographically limited media. The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals, wavelengths of electromagnetic radiation, and dimensions of integrated circuit part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
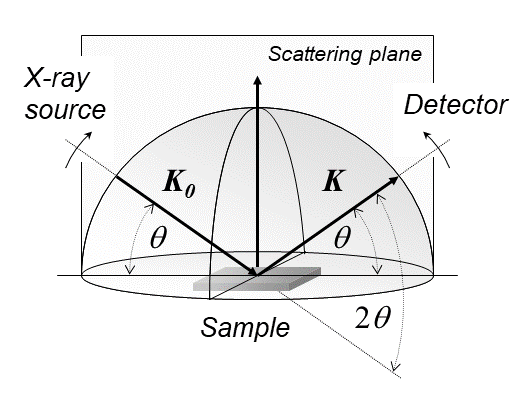
X-ray Diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. The resulting map of the directions of the X-rays far from the sample is called a diffraction pattern. It is different from X-ray crystallography which exploits X-ray diffraction to determine the arrangement of atoms in materials, and also has other components such as ways to map from experimental diffraction measurements to the positions of atoms. This article provides an overview of X-ray diffraction, starting with the early #History, history of x-rays and the discovery that they have the right spacings to be diffracted by crystals. In many cases these diffraction patterns can be #Introduction to x-ray diffraction theory, Interpreted using a single scattering or kinematical theory with conservation of energy (#Ewald's sphere, wave vecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synchrotron
A synchrotron is a particular type of cyclic particle accelerator, descended from the cyclotron, in which the accelerating particle beam travels around a fixed closed-loop path. The strength of the magnetic field which bends the particle beam into its closed path increases with time during the accelerating process, being ''synchronized'' to the increasing kinetic energy of the particles. The synchrotron is one of the first accelerator concepts to enable the construction of large-scale facilities, since bending, beam focusing and acceleration can be separated into different components. The most powerful modern particle accelerators use versions of the synchrotron design. The largest synchrotron-type accelerator, also the largest particle accelerator in the world, is the Large Hadron Collider (LHC) near Geneva, Switzerland, completed in 2008 by the European Organization for Nuclear Research (CERN). It can accelerate beams of protons to an energy of 7 electron volt, teraelectro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a semiconducting material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in conductors the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semimetal
A semimetal is a material with a small energy overlap between the bottom of the Electrical conduction, conduction Electronic band structure, band and the top of the valence band, but they do not overlap in momentum space. According to Band theory, electronic band theory, solids can be classified as Insulator (electricity), insulators, semiconductors, semimetals, or metals. In insulators and semiconductors the filled valence band is separated from an empty conduction band by a band gap. For insulators, the magnitude of the band gap is larger (e.g., > 4 Electronvolt, eV) than that of a semiconductor (e.g., < 4 eV). Because of the slight overlap between the conduction and valence bands, semimetals have no band gap and a small density of states at the Fermi level. A metal, by contrast, has an appreciable density of states at the Fermi level because the conduction band is partially filled. Temperature dependency The insulating/semiconducting st ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem , a place in Finland
{{disambig ...
Chem may refer to: *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" *Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel * CHEM-DT, a Canadian television channel See also * Chemo (other) * Kem (other) *Kemi Kemi (; ; ; ) is a cities of Finland, town and municipalities of Finland, municipality of Finland. It is located approximately from the city of Tornio and the Finland–Sweden border, Swedish border. The distance to Oulu is to the south and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetics (chemistry)
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History The pioneering work of chemical kinetics was done by German chemist Ludwig Wilhelmy in 1850. He experimentally studied the rate of inversion of sucrose and he used integrated rate law for the determination of the reaction kinetics of this reaction. His work was noticed 34 years later by Wilhelm Ostwald. In 1864, Peter Waage and Cato Guldberg published the law of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Voltammetry
In electrochemistry, cyclic voltammetry (CV) is a type of voltammetric measurement where the potential of the working electrode is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles in potential are repeated until the voltammetric trace reaches a cyclic steady state. The current at the working electrode is plotted versus the voltage at the working electrode to yield the cyclic voltammogram (see Figure 1). Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode. Experimental method In cyclic voltammetry (CV), the electrode potential is ramped linearly versus time in cyclical phases. The rate of voltage change over time during each of these phases is known as the scan rate (V/s). In a standar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |