|
Thiazyl Trifluoride
Thiazyl trifluoride is a chemical compound of nitrogen, sulfur, and fluorine, having the formula . It exists as a stable, colourless gas, and is an important precursor to other sulfur-nitrogen-fluorine compounds. It has tetrahedral molecular geometry around the sulfur atom, and is regarded to be a prime example of a compound that has a sulfur-nitrogen triple bond. Preparation can be synthesised by the fluorination of thiazyl fluoride, NSF, with silver(II) fluoride, : : or by the oxidative decomposition of by silver(II) fluoride: : It is also a product of the oxidation of ammonia by . Direct fluorination of mercury difluorosulfinimide (Hg(NSF2)2) does not give thiazyl trifluoride, but instead the isomeric fluoriminosulfur difluoride (F2SNF). Reactions is much more stable than thiazyl fluoride, does not react with ammonia and hydrogen chloride, and only reacts with sodium at 400 °C. However, the fluoride ligands are labile, and can be displaced by secondary amines. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group ''Td'', but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram at left, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonmetal Halides
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less dense) than elements that form metals and are often poor conductors of Thermal conduction, heat and Electrical conductor, electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic. Seventeen elements are widely recognized as nonmetals. Additionally, some or all of six borderline elements (metalloids) are sometimes counted as nonmetals. The two lightest nonmetals, hydrogen and helium, together account for about 98% of the mass of the observable universe. Five nonmetallic elements—hydrogen, carbon, nitrogen, oxygen, and silicon—form the bulk of Earth’s Atmosphere of Earth, atmosphere, biosphere, Earth's crust, crus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
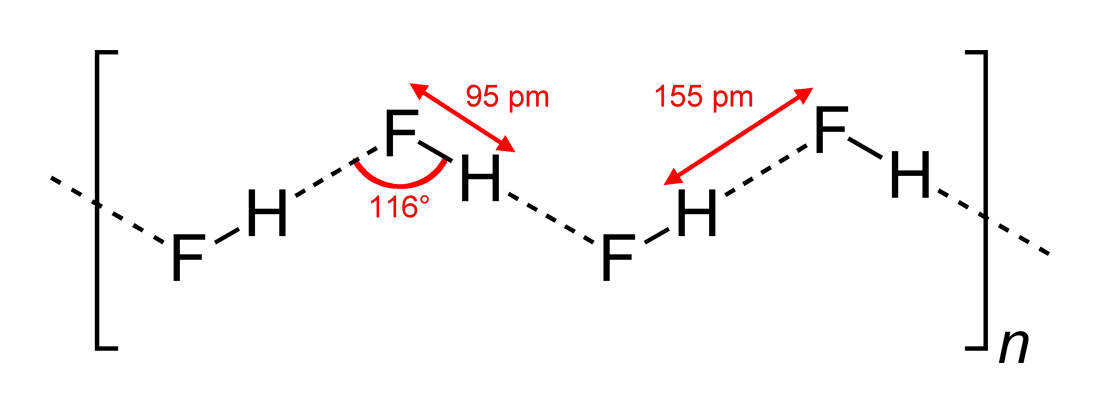
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Fluoride
Carbonyl fluoride is a chemical compound with the formula . It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with ''C''2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°. Preparation and properties Carbonyl fluoride is usually produced as a decomposition product of fluorinated hydrocarbons in the thermal decomposition thereof, for example from trifluoromethanol or tetrafluoromethane in the presence of water: : Carbonyl fluoride can also be prepared by reaction of phosgene with hydrogen fluoride and the fluorination of carbon monoxide, although the latter tends to result in over-fluorination to carbon tetrafluoride. The fluorination of carbon monoxide with silver difluoride is convenient: : Carbonyl fluoride is unstable in the presence of water, hydrolyzing to carbon dioxide and hydrogen fluoride Hydrogen fluoride (fluorane) is an Inorganic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the Abundance of elements in Earth's crust, sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been Leaching (chemistry), leached by the action of water from the Earth, Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in Soap, soap manufac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a Polar-covalent bond, polar covalent bond. The chlorine atom is much more Electronegativity, electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large Molecular dipole moment, dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Decafluoride
Disulfur decafluoride is a chemical compound with the formula . It was discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. The two sulfur atoms are connected by a single bond. In the molecule, the oxidation state of each sulfur atoms is +5, but their valency is 6 (they are hexavalent). is highly toxic, with toxicity four times that of phosgene. It is a colorless liquid with a burnt match smell similar to sulfur dioxide. Production Disulfur decafluoride is produced by photolysis of : : Disulfur decafluoride arises by the decomposition of sulfur hexafluoride. It is produced by the electrical decomposition of sulfur hexafluoride ()—an essentially inert insulator used in high voltage systems such as transmission lines, substations and switchgear. is also made during the production of . Properties The S-S bond dissociation energy is 305 ± 21 kJ/mol, about 80 kJ/mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver(II) Fluoride
Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent. Preparation AgF2 can be synthesized by fluorinating Ag2O with elemental fluorine. Also, at 200 °C (473 K) elemental fluorine will react with AgF or AgCl to produce AgF2. As a strong fluorinating agent, AgF2 should be stored in Teflon or a passivated metal container. It is light sensitive. AgF2 can be purchased from various suppliers, the demand being less than 100 kg/year. While laboratory experiments find use for AgF2, it is too expensive for large scale industry use. In 1993, AgF2 cost between 1000-1400 US dollars per kg. Composition and structure AgF2 is a white crystalline powder, but it is usually black/brown due to impurities. The F/Ag ratio for most samples is < 2, typically approaching 1.75 due to contamination with [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |