|
Tetrafluoroammonium
The tetrafluoroammonium cation (also known as perfluoroammonium) is a positively charged polyatomic ion with chemical formula . It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane , trifluoramine oxide , tetrafluoroborate anion and the tetrafluoroberyllate anion. The tetrafluoroammonium ion forms salts with a large variety of fluorine-bearing anions. These include the bifluoride anion (), tetrafluorobromate (), metal pentafluorides ( where M is Ge, Sn, or Ti), hexafluorides ( where M is P, As, Sb, Bi, or Pt), heptafluorides ( where M is W, U, or Xe), octafluorides (), various oxyfluorides ( where M is W or U; , ), and perchlorate (). Attempts to make the nitrate salt, , were unsuccessful because of quick fluorination: + → + . Structure The geometry of the tetrafluoroammonium ion is tetrahedral, with an estimated nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
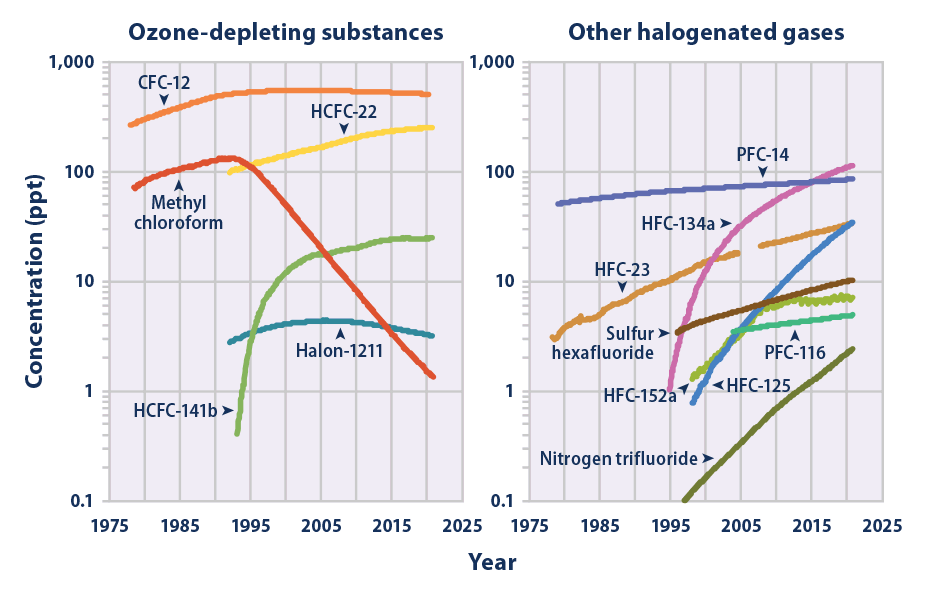
Nitrogen Trifluoride
Nitrogen trifluoride is the inorganic compound with the formula (). It is a colorless, non-flammable, toxic gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period. Synthesis and reactivity Nitrogen trifluoride can be prepared from the elements in the presence of an electric discharge. In 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It is far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perchlorate anion, , which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. As an anion in inorganic and organic chemistry The popularization of has led to decreased use of in the laboratory as a weakly coordinating anion. With organic compounds, especially amine derivatives, forms potentially explosive derivatives. Disadvantages to include its slight sensitivity to hydrolysis and decomposition via loss of a fluoride ligand, whereas does not suffer from these problems. Safety considerations, however, overshadow this inconvenience. With a formula weight of 86.8, BF is also conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name originates from Spanish language, Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 element, group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the Abundance of elements in Earth's crust, rarer elements in Earth's crust, with an average abundance of approximately 5 microgram, μg/kg, making platinum about 30 times rarer than gold. It occurs in some nickel and copper ores along with some Native element mineral, native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury Basin, Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroarsenate
The hexafluoroarsenate (sometimes shortened to fluoroarsenate) anion is a chemical species with formula . Hexafluoroarsenate is relatively inert, being the conjugate base of the notional superacid hexafluoroarsenic acid (). Synthesis The first undisputed synthesis is due to Otto Ruff, Kurt Stäuber and Hugo Graf, who began with the lower-valent arsenic trifluoride, using silver(I) fluoride as both a fluorine source and oxidant: cites but discounts it as describing an implausibly easy synthesis with a hydrolyzable product. : In the following reaction, one mole of arsenic trifluoride, three moles of silver fluoride, and one mole of nitrosyl chloride are reacted to produce one mole of nitrosyl hexafluoroarsenate, one mole of silver chloride, and two moles of elemental silver. Modern syntheses usually begin with arsenic pentafluoride (), which abstracts fluoride from common donors, such as hydrogen fluoride () or ''cis''- difluorodiazine (). Although the hexafluoroarsenate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Pentafluoride
Antimony pentafluoride is the inorganic compound with the formula Sb F5. This colorless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds. Preparation Antimony pentafluoride is prepared by the reaction of antimony pentachloride with anhydrous hydrogen fluoride:Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf "Antimony and Antimony Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim :SbCl5 + 5 HF → SbF5 + 5 HCl It can also be prepared from antimony trifluoride and fluorine. Structure and chemical reactions In the gas phase, SbF5 adopts a trigonal bipyramidal structure of D3h point group symmetry (see picture). The material adopts a more complicated structure in the liquid and solid states. The liquid contains polymers whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important Reagent, chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picometre
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry, and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of which ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group ''Td'', but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram at left, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of Resonance (chemistry), resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |