|
Sugar Phosphates
Sugar phosphates (sugars that have added or substituted phosphate groups) are often used in biological systems to store or transfer energy. They also form the backbone for DNA and RNA. Sugar phosphate backbone geometry is altered in the vicinity of the modified nucleotides. Examples include: * Dihydroxyacetonephosphate * Glucose-6-phosphate * Phytic acid * Teichoic acid Electronic structure of the sugar-phosphate backbone The sugar-phosphate backbone has multiplex electronic structure and the electron delocalisation complicates its theoretical description. Some part of the electronic density is delocalised over the whole backbone and the extent of the delocalisation is affected by backbone conformation due to hyper-conjugation effects. Hyper-conjugation arises from donor-acceptor interactions of localised orbitals in 1,3 positions. Phosphodiesters in DNA and RNA The phosphodiester backbone of DNA and RNA consists of pairs of deoxyribose or ribose sugars linked by phosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleic Acids
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is the ribose derivative deoxyribose, the polymer is DNA. Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells and make up the genetic material. Nucleic acids are found in abundance in all living things, where they create, encode, and then store information of every living cell of every life-form on Earth. In turn, they function to transmit and express that information inside and outside the cell nucleus to the interior operations of the cell and ultimately to the next generation of each living organism. The encoded information is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Cleavage
In chemistry, bond cleavage, or bond fission, is the splitting of chemical bonds. This can be generally referred to as dissociation when a molecule is cleaved into two or more fragments. In general, there are two classifications for bond cleavage: ''homo''lytic and ''hetero''lytic, depending on the nature of the process. The triplet and singlet excitation energies of a sigma bond can be used to determine if a bond will follow the homolytic or heterolytic pathway. A metal−metal sigma bond is an exception because the bond's excitation energy is extremely high, thus cannot be used for observation purposes. In some cases, bond cleavage requires catalysts. Due to the high bond-dissociation energy of C-H bonds, around , a large amount of energy is required to cleave the hydrogen atom from the carbon and bond a different atom to the carbon. Homolytic cleavage In homolytic cleavage, or homolysis, the two electrons in a cleaved covalent bond are divided equally between t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolytic
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small bits of nucleic acids can be manufactured as single-stranded molecules with any user-specified sequence, and so are vital for artificial gene synthesis, polymerase chain reaction (PCR), DNA sequencing, molecular cloning and as molecular probes. In nature, oligonucleotides are usually found as small RNA molecules that function in the regulation of gene expression (e.g. microRNA), or are degradation intermediates derived from the breakdown of larger nucleic acid molecules. Oligonucleotides are characterized by the sequence of nucleotide residues that make up the entire molecule. The length of the oligonucleotide is usually denoted by " -mer" (from Greek ''meros'', "part"). For example, an oligonucleotide of six nucleotides (nt) is a hexamer, while one of 25 nt w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Achiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, ''enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter how t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Peptide
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis (long peptides) in living organisms occurs in the opposite direction. The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below). Solution-phase synthesis retains its usefulness in large-scale production of peptides for industrial purposes howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
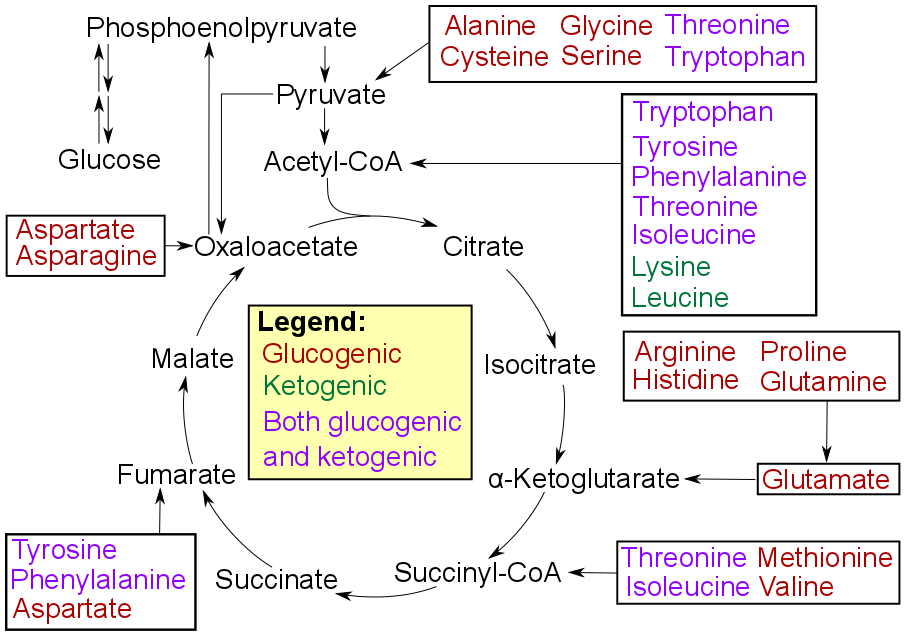
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal. In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Ka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non- carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen ( glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels ( hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degradation (chemistry)
Degradation may refer to: Science * Degradation (geology), lowering of a fluvial surface by erosion * Degradation (telecommunications), of an electronic signal * Biodegradation of organic substances by living organisms * Environmental degradation in ecology * Land degradation, a process in which the value of the biophysical environment is affected by a combination of human-induced processes acting upon the land * Polymer degradation, as plastics age Other * Elegant degradation, gradual rather than sudden * Graceful degradation, in a fault-tolerant system * Degradation (knighthood), revocation of knighthood * Cashiering, whereby a military officer is dismissed for misconduct * Reduction in rank, whereby a military officer is reduced to a lower rank for misconduct * Degradation, the former ceremony of defrocking a disgraced priest * ''Degradation'', a song by the Violent Femmes, from ''Add It Up (1981–1993) ''Add It Up (1981–1993)'' is a compilation album released by Violent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide Phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the reduced form of NADP. NADP differs from NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase can convert NADPH back to NADH to maintain a balance. Some forms of the NAD+ kinas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as '' catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |