|
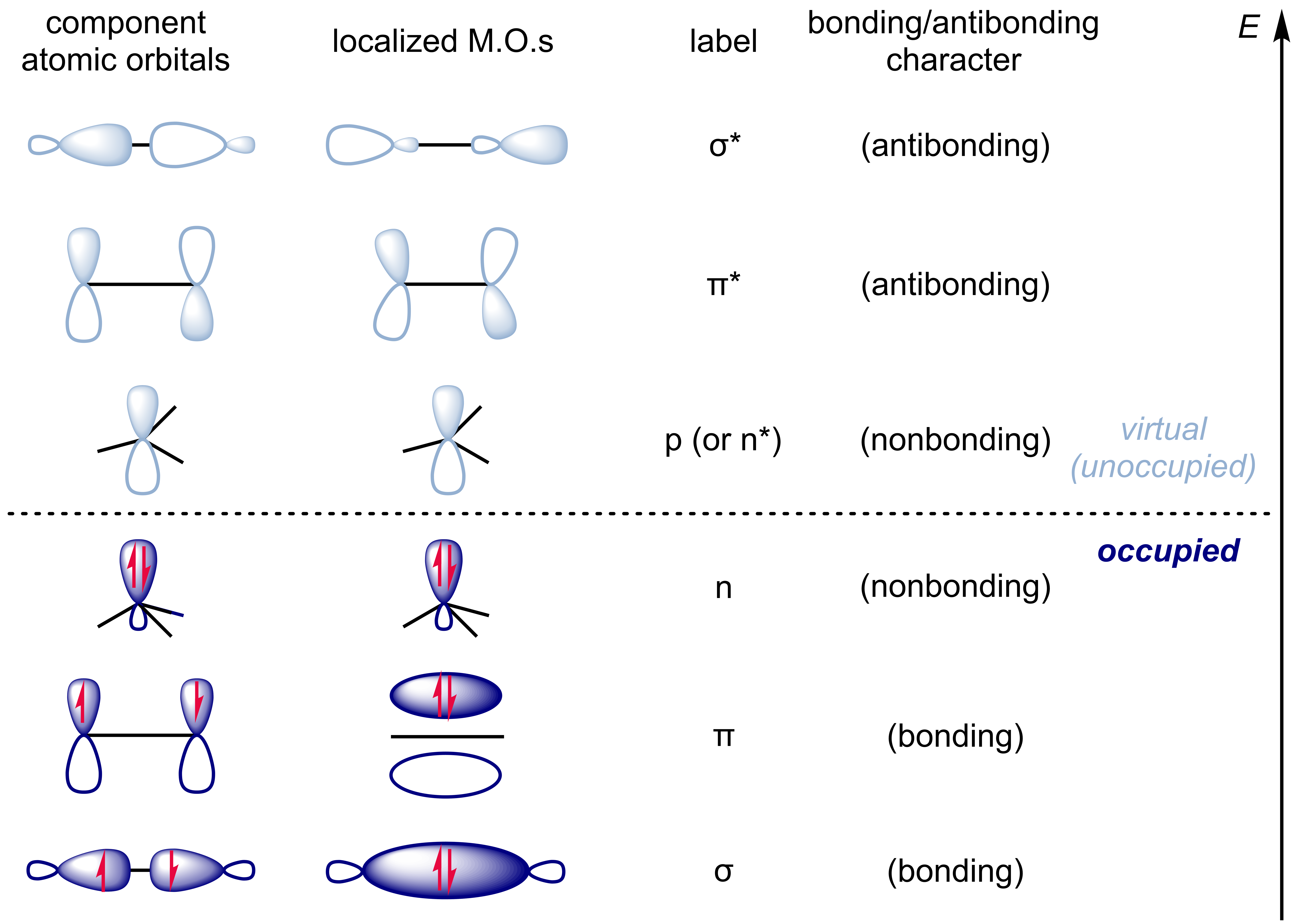
Sigma-pi And Equivalent-orbital Models
The σ-π model and equivalent-orbital model refer to two possible representations of molecules in valence bond theory. The σ-π model differentiates bonds and lone pairs of σ symmetry from those of π symmetry, while the equivalent-orbital model hybridizes them. The σ-π treatment takes into account molecular symmetry and is better suited to interpretation of aromatic molecules (Hückel's rule), although computational calculations of certain molecules tend to optimize better under the equivalent-orbital treatment. The two representations produce the same total electron density and are related by a unitary transformation of the occupied molecular orbitals; different localization procedures yield either of the two. Two equivalent orbitals ''h'' and ''h''' can be constructed by taking linear combinations ''h'' = ''c''1σ + ''c''2π and ''h''' = ''c''1σ – ''c''2π for an appropriate choice of coefficients ''c''1 and ''c''2. In a 1996 review, Kenneth B. Wiberg concluded that "al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Bond Theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. In contrast, molecular orbital theory has orbitals that cover the whole molecule. History Lothar Meyer in his 1864 book, ''Die modernen Theorien der Chemie'', contained an early version of the periodic table containing 28 elements, classified elements into six families by their valence—for the first time, elements had been grouped according to their valence. Works on organizing the elements by atomic weight, until then had been stymied by the widespread use of equivalent weights for the elements, rather than atomic weights. In 1916, G. N. Lewis proposed that a chemical bond forms by the interaction of two shared bonding electrons, with the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hückel's Rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4''n'' + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time. In agreement with the Möbius–Hückel concept, a cyclic ring molecule follows Hückel's rule when the number of its π-electrons equals 4''n'' + 2, although clearcut examples are really only established for values of ''n'' = 0 up to about ''n'' = 6. Hückel's rule was originally based on calculations using the Hückel method, although it can also be justified by considering a particle in a ring system, by the LCAO method and by the Pariser–Parr–Pople method. Aromatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unitary Transformation
In mathematics, a unitary transformation is a transformation that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation. Formal definition More precisely, a unitary transformation is an isomorphism between two inner product spaces (such as Hilbert spaces). In other words, a ''unitary transformation'' is a bijective function U : H \to H_2\, between two inner product spaces, H and H_2, such that \langle Ux, Uy \rangle_ = \langle x, y \rangle_ \quad \text x, y \in H. Properties A unitary transformation is an isometry In mathematics, an isometry (or congruence, or congruent transformation) is a distance-preserving transformation between metric spaces, usually assumed to be bijective. The word isometry is derived from the Ancient Greek: ἴσος ''isos'' ..., as one can see by setting x=y in this formula. Unitary operator In the case when H_1 and H_2 are the same space, a unitary tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Localized Molecular Orbitals
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The local ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kenneth B
Kenneth is an English given name and surname. The name is an Anglicised form of two entirely different Gaelic personal names: ''Cainnech'' and '' Cináed''. The modern Gaelic form of ''Cainnech'' is ''Coinneach''; the name was derived from a byname meaning "handsome", "comely". A short form of ''Kenneth'' is '' Ken''. Etymology The second part of the name ''Cinaed'' is derived either from the Celtic ''*aidhu'', meaning "fire", or else Brittonic ''jʉ:ð'' meaning "lord". People :''(see also Ken (name) and Kenny)'' Places In the United States: * Kenneth, Indiana * Kenneth, Minnesota * Kenneth City, Florida Kenneth City is a town in southern Pinellas County, Florida, between St. Petersburg and Pinellas Park, United States. The population was 4,980 at the 2010 US Census. History Kenneth City was founded in 1957 by Sidney Colen, a local developer, ... In Scotland: * Inch Kenneth, an island off the west coast of the Isle of Mull Other * " What's the Frequency, Kenneth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ian Fleming (chemist)
Ian Fleming (born 1935) is an English organic chemist, and an emeritus professor of the University of Cambridge, and an emeritus fellow of Pembroke College, Cambridge. He was the first to determine the full structure of chlorophyll (in 1967) and was involved in the development of the synthesis of cyanocobalamin by Robert Burns Woodward. He has made major contributions to the use of organosilicon compounds for stereospecific syntheses; reactions which have found application in the synthesis of natural compounds. He is also a prolific author, and has written a number of textbooks, encyclopedia chapters and influential review articles. Life and research Ian Fleming was born August 4, 1935, in Staffordshire and grew up in Stourbridge, Worcestershire. He received a B.A. in 1959 and a Ph.D. in 1962, both from Pembroke College, Cambridge. His post-doctoral studies were done at Harvard University with R.B. Woodward on the synthesis of vitamin B12. He has made advances in the top ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 greatest scientists of all time, and as of 2000, he was rated the 16th most important scientist in history. For his scientific work, Pauling was awarded the Nobel Prize in Chemistry in 1954. For his peace activism, he was awarded the Nobel Peace Prize in 1962. He is one of five people to have won more than one Nobel Prize (the others being Marie Curie, John Bardeen, Frederick Sanger and Karl Barry Sharpless). Of these, he is the only person to have been awarded two unshared Nobel Prizes, and one of two people to be awarded Nobel Prizes in different fields, the other being Marie Curie. Pauling was one of the founders of the fields of quantum chemistry and molecular biology. His contributions to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erich Hückel
Erich Armand Arthur Joseph Hückel (August 9, 1896, Berlin – February 16, 1980, Marburg) was a German physicist and physical chemist. He is known for two major contributions: *The Debye–Hückel theory of electrolytic solutions *The Hückel method of approximate molecular orbital (MO) calculations on π electron systems. Hückel was born in the Charlottenburg suburb of Berlin. He studied physics and mathematics from 1914 to 1921 at the University of Göttingen. On receiving his doctorate, he became an assistant at Göttingen, but soon became an assistant to Peter Debye at Zürich. It was there that he and Debye developed their theory (the Debye–Hückel theory, in 1923) of electrolytic solutions, elucidating the behavior of strong electrolytes by considering interionic forces, in order to account for their electrical conductivity and their thermodynamic activity coefficients. After spending 1928 and 1929 in England and Denmark, working briefly with Niels Bohr, Hückel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma Bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is symmetrical with respect to rotation about the bond axis. By this definition, common forms of sigma bonds are s+s, pz+pz, s+pz and dz2+dz2 (where z is defined as the axis of the bond or the internuclear axis). Quantum theory also indicates that molecular orbitals (MO) of identical symmetry actually mix or ''hybridize''. As a practical consequence of this mixing of diatomic molecules, the wavefunctions s+s and pz+pz molecular orbitals become blended. The extent of this mixing (or hybridization or blending) depends on the relative energies of the MOs of like symmetry. For homodiatomics ( homonuclear diatomic molecules), bonding σ orbitals have no nodal planes at which the wavefunction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |