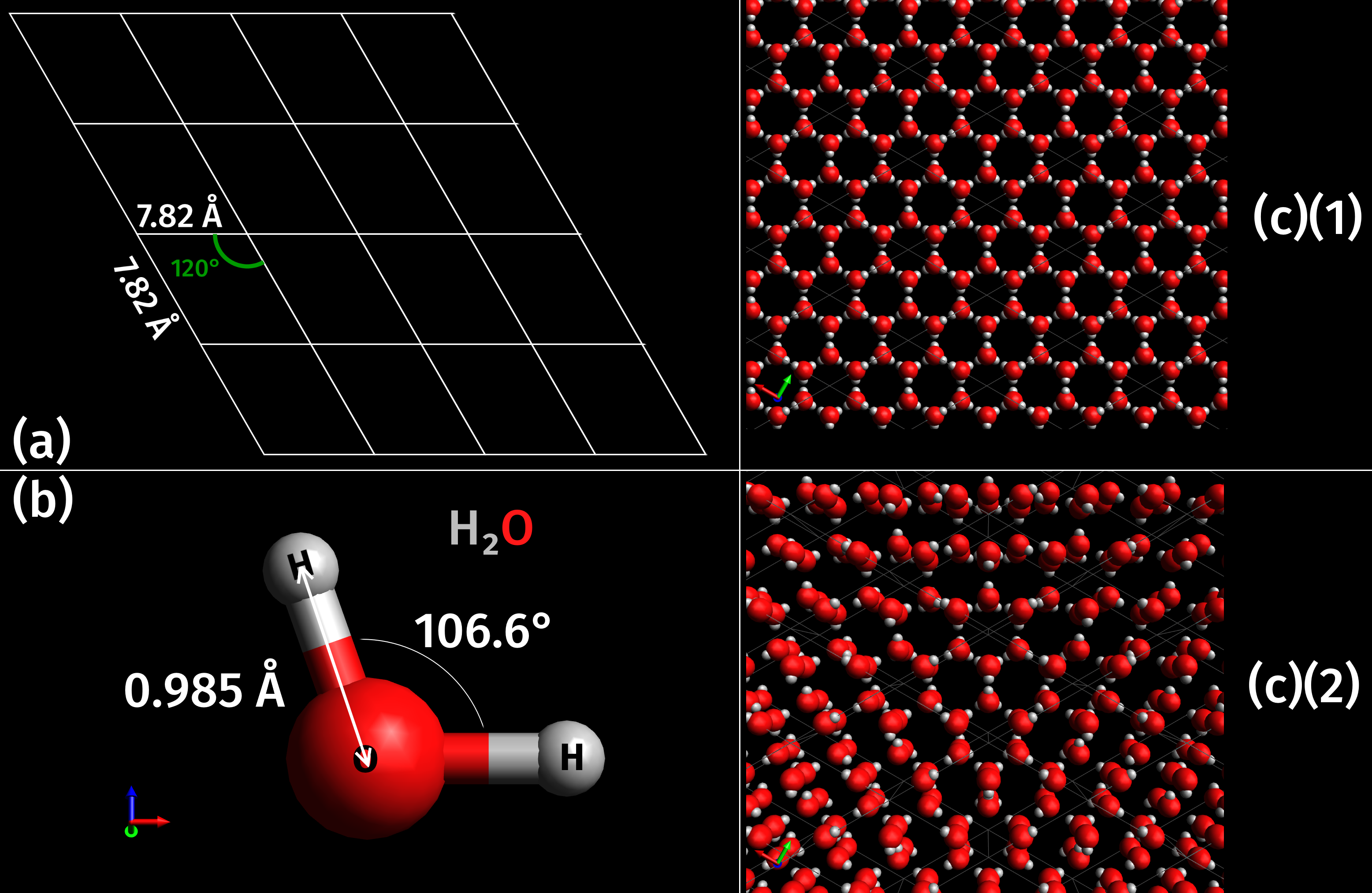
|
Supercooled Water
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. Per the established international definition, supercooling means ''‘cooling a substance below the normal freezing point without solidification’.'' IIR International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.php ASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to . Supercooled water can occur naturally, for example in the atmosphere, animals or plants. This phenomenon was first identified in 1724 by Daniel Gabriel Fahrenheit, while developing Fahrenheit scale. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solute
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, tempe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing-point Depression
Freezing-point depression is a drop in the maximum temperature at which a substance freezing, freezes, caused when a smaller amount of another, non-Volatility (chemistry), volatile substance is added. Examples include adding salt into water (used in ice cream makers and for De-icing#Chemical de-icers, de-icing roads), Alcohol (chemistry), alcohol in water, Ethylene glycol, ethylene or propylene glycol in water (used in antifreeze in cars), adding copper to molten silver (used to make Solder#Hard_solder, solder that flows at a lower temperature than the silver pieces being joined), or the mixing of two solids such as impurities into a finely powdered drug. In all cases, the substance added/present in smaller amounts is considered the solute, while the original substance present in larger quantity is thought of as the solvent. The resulting liquid solution or solid-solid mixture has a lower Melting point, freezing point than the pure solvent or solid because the chemical potentia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superheating
In thermodynamics, superheating (sometimes referred to as boiling retardation, or boiling delay) is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. This is a so-called ''metastable state'' or '' metastate'', where boiling might occur at any time, induced by external or internal effects.Debenedetti, P.G.Metastable Liquids: Concepts and Principles; Princeton University Press: Princeton, NJ, USA, 1996.Maris, H., Balibar, S. (2000"Negative Pressures and Cavitation in Liquid Helium"Physics Today 53, 29 Superheating is achieved by heating a homogeneous substance in a clean container, free of nucleation sites, while taking care not to disturb the liquid. This may occur by microwaving water in a very smooth container. Disturbing the water may cause an unsafe eruption of hot water and result in burns. Cause Water is said to "boil" when bubbles of water vapor grow without bound, bursting at the surface. For a vapor bubble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Journal Of Experimental Biology
''Journal of Experimental Biology'' (formerly ''The British Journal of Experimental Biology)'' is a peer-reviewed scientific journal in the field of comparative physiology and integrative biology. It is published by The Company of Biologists. The journal is partnered with Publons and has two-way integration with bioRxiv. ''Journal of Experimental Biology'' is now a hybrid journal and publishes 24 issues a year. Content over six months old is free to read. History ''The'' ''British Journal of Experimental Biology'' was established in Edinburgh in 1923 (''Br. J. Exp. Biol.'': ). It was published by Oliver and Boyd and edited by F. A. E. Crew with an Editorial Board of nine members, including Julian Huxley. When the journal ran into financial trouble, George Parker Bidder II, the founder of The Company of Biologists, rescued it in 1925. Sir James Gray was appointed as the journal's first Editor-in-Chief in 1925 and the journal was renamed ''The Journal of Experimental Biol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point Apparatus
A melting-point apparatus is a scientific instrument used to determine the melting point of a substance. Some types of melting-point apparatuses include the Thiele tube, Fisher-Johns apparatus, Gallenkamp (Electronic) melting-point apparatus and automatic melting-point apparatus. Design While the outward designs of apparatuses can vary greatly, most apparatuses use a sample loaded into a sealed capillary tube (''melting-point tube''), which is then placed in the apparatus. The sample is then heated, either by a heating block or an oil bath, and as the temperature increases, the sample is observed to determine when the phase change from solid to liquid occurs. The operator of the apparatus records the temperature range starting with the initial phase-change temperature and ending with the completed phase-change temperature. The temperature range that is determined can then be averaged to gain the melting point of the sample being examined. Apparatuses usually have a control p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various #Units, units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the International System of Units, SI unit of pressure, the Pascal (unit), pascal (Pa), for example, is one newton (unit), newton per square metre (N/m2); similarly, the Pound (force), pound-force per square inch (Pound per square inch, psi, symbol lbf/in2) is the traditional unit of pressure in the imperial units, imperial and United States customary units, US customary systems. Pressure ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing Rain
Freezing rain is rain maintained at temperatures below melting point, freezing by the ambient air mass that causes freezing on contact with surfaces. Unlike rain and snow mixed, a mixture of rain and snow or ice pellets, freezing rain is made entirely of liquid droplets. The raindrops become supercooling, supercooled while passing through a sub-freezing layer of air hundreds of meters above the ground, and then freeze upon impact with any surface they encounter, including the ground, trees, electrical wires, aircraft, and automobiles. The resulting ice, called glaze (ice), glaze ice, can accumulate to a thickness of several centimeters and cover all exposed surfaces. The METAR code for freezing rain is FZRA. A storm that produces a significant thickness of glaze ice from freezing rain is often referred to as an ice storm. Although these storms are not particularly violent, freezing rain is notorious for causing travel problems on roadways, breaking tree limbs, and downing power l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Protection System
Ice is water that is frozen into a solid state, typically forming at or below temperatures of 0 ° C, 32 ° F, or 273.15 K. It occurs naturally on Earth, on other planets, in Oort cloud objects, and as interstellar ice. As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. Virtually all of the ice on Earth is of a hexagonal crystalline structure denoted as ''ice Ih'' (spoken as "ice one h"). Depending on temperature and pressure, at least nineteen phases ( packing geometries) can exist. The most common phase transition to ice Ih occurs when liquid water is cooled below (, ) at standard atmospheric pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form. Interstellar ice is overwhelmingly low-density amorphous ice (LD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |