|
Stonustoxin
Stonustoxin (SNTX) is an extremely potent Cytolysin, cytolytic toxin of the perforin-like superfamily. It is mainly found in stonefish (Synanceia). The name Stonustoxin is an abbreviation of ''STO''nefish ''N''ational ''U''niversity of ''S''ingapore. History Little is known about the biological activity and composition of marine Venomous fish, fish venoms, due to the difficulties in obtaining, storing and extracting venom samples. The National University of Singapore performed the first purification of the stonefish venom, because stonefish stings have been responsible for a number of deaths and severe poisoning cases in the local area. The molecular weight was determined by high performance liquid chromatography at pH 7.0 using 10 mM sodium phosphate buffer (chemistry), buffer with 0.2M sodium sulfate in a Gel permeation chromatography, gel permeation column at a flow rate of 1.0 ml/min. Structure The exact conformation of stonustoxin has been described. Stonustoxin is a heterod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytolysin
Cytolysin refers to the substance secreted by microorganisms, plants or animals that is specifically toxic to individual cells, in many cases causing their dissolution through lysis. Cytolysins that have a specific action for certain cells are named accordingly. For instance, the cytolysins responsible for the destruction of red blood cells, thereby liberating hemoglobins, are named '' hemolysins'', and so on. Cytolysins may be involved in immunity as well as in venoms. Hemolysin is also used by certain bacteria, such as '' Listeria monocytogenes'', to disrupt the phagosome membrane of macrophages and escape into the cytoplasm of the cell. History and background The term "Cytolysin" or "Cytolytic toxin" was first introduced by Alan Bernheimer to describe membrane damaging toxins (MDTs) that have cytolytic effects to cells. The first kind of cytolytic toxin discovered have hemolytic effects on erythrocytes of certain sensitive species, such as Human. For this reason " Hemol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respiratory Distress
Shortness of breath (SOB), also medically known as dyspnea (in AmE) or dyspnoea (in BrE), is an uncomfortable feeling of not being able to breathe well enough. The American Thoracic Society defines it as "a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity", and recommends evaluating dyspnea by assessing the intensity of its distinct sensations, the degree of distress and discomfort involved, and its burden or impact on the patient's activities of daily living. Distinct sensations include effort/work to breathe, chest tightness or pain, and "air hunger" (the feeling of not enough oxygen). The tripod position is often assumed to be a sign. Dyspnea is a normal symptom of heavy physical exertion but becomes pathological if it occurs in unexpected situations, when resting or during light exertion. In 85% of cases it is due to asthma, pneumonia, cardiac ischemia, interstitial lung disease, congestive heart f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edema
Edema, also spelled oedema, and also known as fluid retention, dropsy, hydropsy and swelling, is the build-up of fluid in the body's tissue. Most commonly, the legs or arms are affected. Symptoms may include skin which feels tight, the area may feel heavy, and joint stiffness. Other symptoms depend on the underlying cause. Causes may include venous insufficiency, heart failure, kidney problems, low protein levels, liver problems, deep vein thrombosis, infections, angioedema, certain medications, and lymphedema. It may also occur after prolonged sitting or standing and during menstruation or pregnancy. The condition is more concerning if it starts suddenly, or pain or shortness of breath is present. Treatment depends on the underlying cause. If the underlying mechanism involves sodium retention, decreased salt intake and a diuretic may be used. Elevating the legs and support stockings may be useful for edema of the legs. Older people are more commonly affected. The wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypotension
Hypotension is low blood pressure. Blood pressure is the force of blood pushing against the walls of the arteries as the heart pumps out blood. Blood pressure is indicated by two numbers, the systolic blood pressure (the top number) and the diastolic blood pressure (the bottom number), which are the maximum and minimum blood pressures, respectively. A systolic blood pressure of less than 90 millimeters of mercury (mmHg) or diastolic of less than 60 mmHg is generally considered to be hypotension. Different numbers apply to children. However, in practice, blood pressure is considered too low only if noticeable symptoms are present. Symptoms include dizziness or lightheadedness, confusion, feeling tired, weakness, headache, blurred vision, nausea, neck or back pain, an irregular heartbeat or feeling that the heart is skipping beats or fluttering, or fainting. Hypotension is the opposite of hypertension, which is high blood pressure. It is best understood as a physiolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyaluronidase
Hyaluronidases are a family of enzymes that catalyse the degradation of hyaluronic acid (HA). Karl Meyer classified these enzymes in 1971, into three distinct groups, a scheme based on the enzyme reaction products. The three main types of hyaluronidases are two classes of eukaryotic endoglycosidase hydrolases and a prokaryotic lyase-type of glycosidase. In humans, there are five functional hyaluronidases: HYAL1, HYAL2, HYAL3, HYAL4 and HYAL5 (also known as SPAM1 or PH-20); plus a pseudogene, HYAL6 (also known as HYALP1). The genes for HYAL1-3 are clustered in chromosome 3, while HYAL4-6 are clustered in chromosome 7. HYAL1 and HYAL2 are the major hyaluronidases in most tissues. GPI-anchored HYAL2 is responsible for cleaving high-molecular weight HA, which is mostly bound to the CD44 receptor. The resulting HA fragments of variable size are then further hydrolized by HYAL1 after being internalized into endo-lysosomes; this generates HA oligosaccharides. According to their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
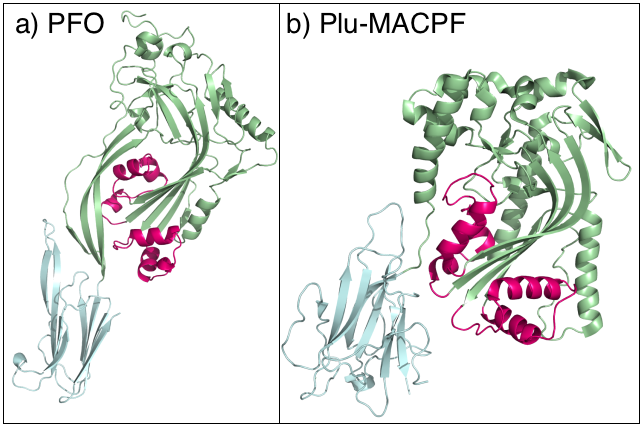
MACPF
The Membrane Attack Complex/Perforin (MACPF) superfamily, sometimes referred to as the MACPF/CDC superfamily, is named after a domain that is common to the membrane attack complex (MAC) proteins of the complement system (C6, C7, C8α, C8β and C9) and perforin (PF). Members of this protein family are pore-forming toxins (PFTs). In eukaryotes, MACPF proteins play a role in immunity and development. Archetypal members of the family are complement C9 and perforin, both of which function in human immunity. C9 functions by punching holes in the membranes of Gram-negative bacteria. Perforin is released by cytotoxic T cells and lyses virally infected and transformed cells. In addition, perforin permits delivery of cytotoxic proteases called granzymes that cause cell death. Deficiency of either protein can result in human disease. Structural studies reveal that MACPF domains are related to cholesterol-dependent cytolysins (CDCs), a family of pore forming toxins previously t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
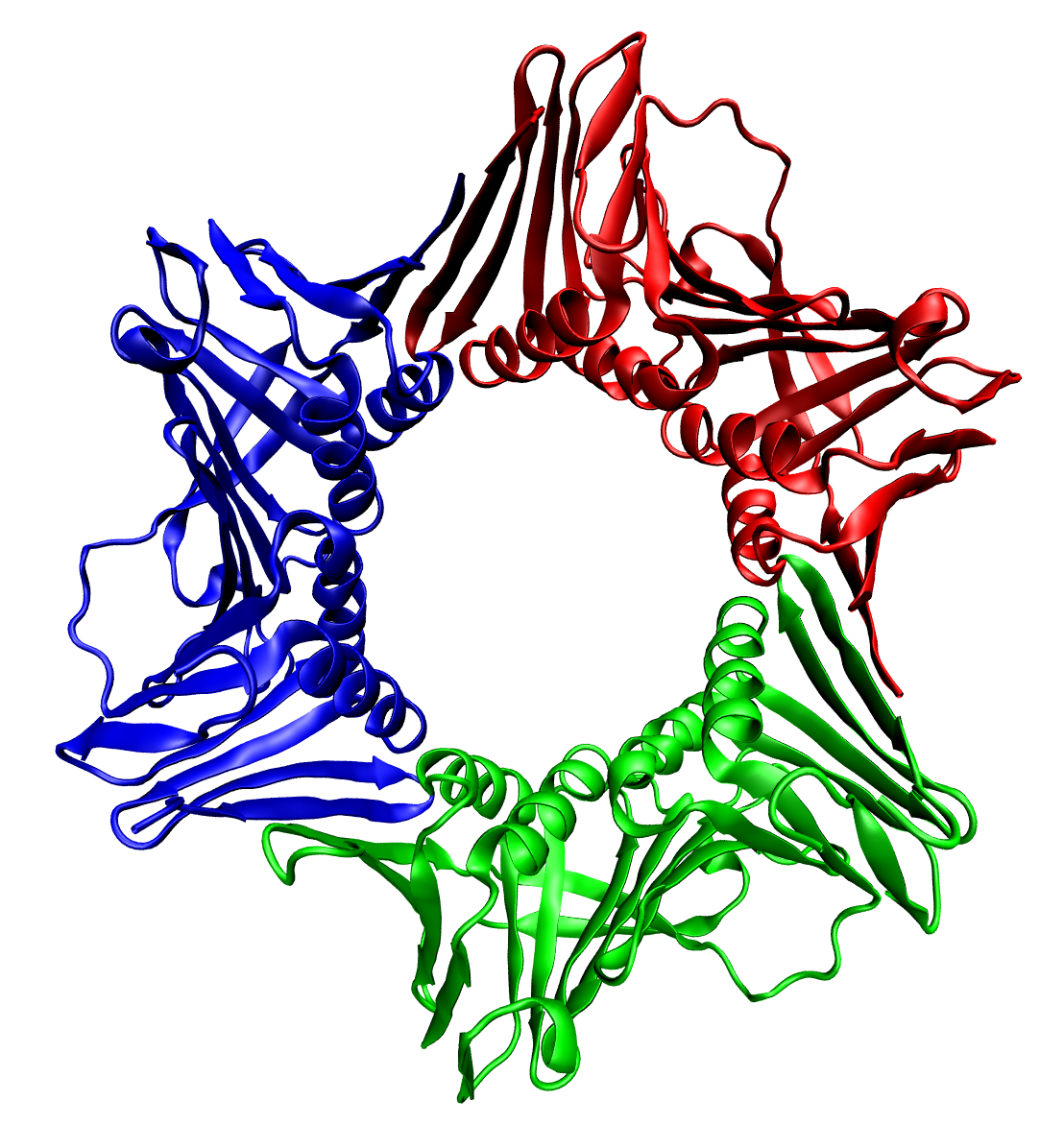
Quaternary Structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors. Description and examples Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of the protein subunits wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellular Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |